Your Location:Home >Products >Fine chemicals >63478-76-2
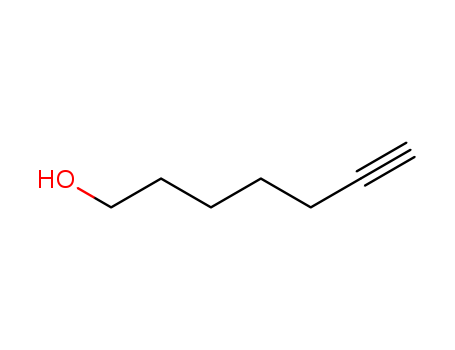

Product Details
|
Chemical Properties |
6-Heptyn-1-ol is Pale Yellow Oil |
|
Uses |
6-Heptyn-1-ol reagents used in the preparation of many cyclic compounds. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 49, p. 5175, 1984 DOI: 10.1021/jo00200a032 |
InChI:InChI=1/C7H12O/c1-2-3-4-5-6-7-8/h1,8H,3-7H2
A new diacetylene containing photopolyme...
The combination of ring-closing metathes...
The total syntheses of the enantiomers o...
A unified synthetic approach to the basi...
In this paper we present a study into th...
A regio- and diastereoselective copper-c...
In Mycobacterium tuberculosis, mycolic a...
The first total synthesis of the tetrasu...
A series of bis(4-pentylpyridinium) comp...
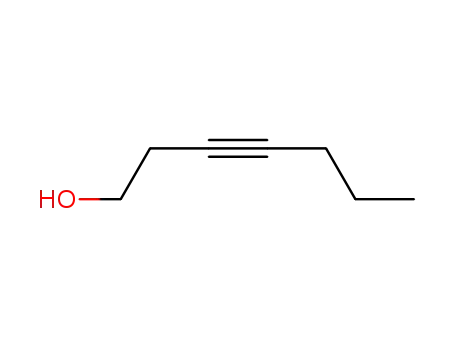
3-heptyn-1-ol

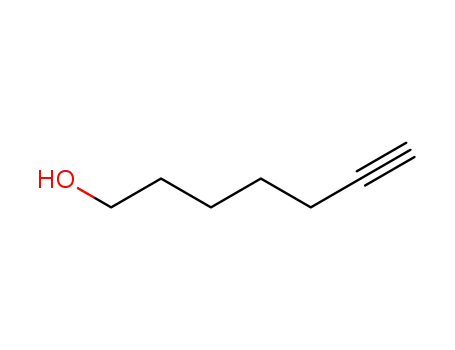
1-hydroxy-6-heptyne
| Conditions | Yield |
|---|---|
|
With sodium hydride; Trimethylenediamine; at 20 ℃; for 0.666667h;
|
100% |
|
With potassium tert-butylate; sodium hydride; Trimethylenediamine; In mineral oil; at 20 - 70 ℃; for 3h;
|
99% |
|
With potassium tert-butylate; lithium; Trimethylenediamine; at 20 ℃; for 2h;
|
97% |
|
With potassium hydride; Trimethylenediamine; at 20 ℃; Inert atmosphere;
|
97% |
|
With sodium hydride; ethylenediamine; In mineral oil; at 10 ℃;
|
93% |
|
With sodium amide; Trimethylenediamine; In various solvent(s); at 80 ℃; for 2h;
|
90% |
|
With sodium hydride; ethylenediamine; In various solvent(s); at 65 ℃; for 1h;
|
83% |
|
With sodium hydride; ethylenediamine; at 65 ℃; for 1h;
|
83% |
|
With sodium hydride; Trimethylenediamine; at 70 ℃; for 1.5h;
|
83% |
|
With sodium hydride; In ethylene-1,2-diamine; Inert atmosphere;
|
81% |
|
With potassium tert-butylate; lithium; Trimethylenediamine;
|
80% |
|
With potassium salt of 1,3-diaminopropane; for 1.5h; Ambient temperature; sonicated;
|
78% |
|
3-heptyn-1-ol; With sodium hydride; ethylenediamine; at 45 - 60 ℃; for 1h; Inert atmosphere;
With hydrogenchloride; In water; ethylenediamine; at 0 ℃; Inert atmosphere;
|
78% |
|
With sodium hydride; In ethylenediamine; at 0 - 60 ℃;
|
72% |
|
With sodium amide; Trimethylenediamine; at 80 ℃; for 2h;
|
71% |
|
With sodium hydride; ethylenediamine; at 60 ℃;
|
68% |
|
With sodium hydride; ethylenediamine; In mineral oil; at 45 - 60 ℃; Inert atmosphere;
|
68% |
|
With sodium hydride; ethylenediamine; Inert atmosphere;
|
64% |
|
3-heptyn-1-ol; With sodium hydride; ethylenediamine; at 20 - 65 ℃; Inert atmosphere;
With hydrogenchloride; In water; for 4h; Inert atmosphere;
|
60% |
|
With lithium; In Trimethylenediamine; at 40 - 60 ℃; Inert atmosphere;
|
48% |
|
With lithium; Trimethylenediamine; at 40 - 60 ℃;
|
48% |
|
With sodium hydride; Trimethylenediamine; In mineral oil; pentane; at 0 - 70 ℃; for 1.5h;
|
21% |
|
With potassium salt of 1,3-diaminopropane; Trimethylenediamine;
|
|
|
With potassium salt of 1,3-diaminopropane; ammonium chloride; Yield given. Multistep reaction; 1.)1,3-diaminopropane, 4 h, R.T., 2.)ether, 0 deg C;
|
|
|
With sodium hydride; at 20 - 70 ℃; for 2h; Inert atmosphere;
|
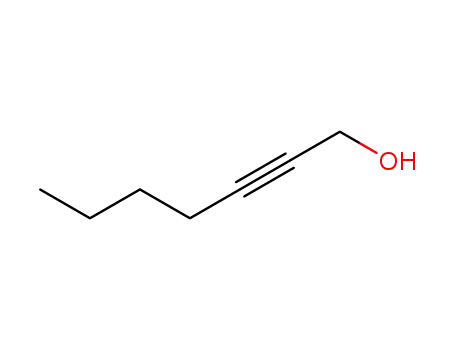
hep-2-yn-1-ol


1-hydroxy-6-heptyne
| Conditions | Yield |
|---|---|
|
With potassium hydride; 1,2-diaminopropan; In tetrahydrofuran; mineral oil; at 20 ℃; for 24h; Inert atmosphere;
|
99% |
|
With potassium salt of 1,3-diaminopropane; In tetrahydrofuran; at 0 ℃; for 0.5h;
|
97% |
|
With potassium tert-butylate; lithium; Trimethylenediamine; at 20 ℃; for 3h; Inert atmosphere;
|
90% |
|
With potassium salt of 1,3-diaminopropane;
|
88% |
|
With sodium hydride; ethylenediamine; at 60 ℃;
|
88% |
|
With potassium salt of 1,3-diaminopropane;
|
87% |
|
With potassium tert-butylate; lithium; ethylenediamine; for 3h;
|
83% |
|
With lithium 2-aminoethylamide; In ethylenediamine; for 0.333333h; amide-hydrocarbon ratio 7:1;
|
82% |
|
With potassium hydride; Trimethylenediamine; for 2h; cooling;
|
79% |
|
hep-2-yn-1-ol; With potassium tert-butylate; lithium; Trimethylenediamine; at 20 ℃; for 1h;
With water;
|
74% |
|
With potassium salt of 1,3-diaminopropane; Trimethylenediamine;
|
73% |
|
With potassium hydride; ethylenediamine; at 20 ℃;
|
70% |
|
With potassium hydride; In various solvent(s); at 10 - 15 ℃; for 1.5h;
|
67% |
|
With sodium hydride; ethylenediamine; at 60 - 65 ℃; for 3h;
|
64% |
|
With sodium hydride; ethylenediamine; In mineral oil; at 0 - 70 ℃;
|
61% |
|
With potassium hydride; Trimethylenediamine;
|
46% |
|
With potassium salt of 1,3-diaminopropane; at 20 ℃; for 1h;
|
1.09 g |
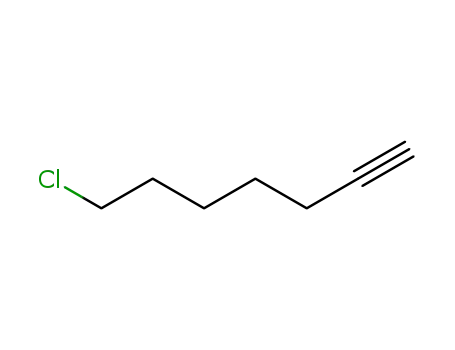
chloro-1 heptyne-6
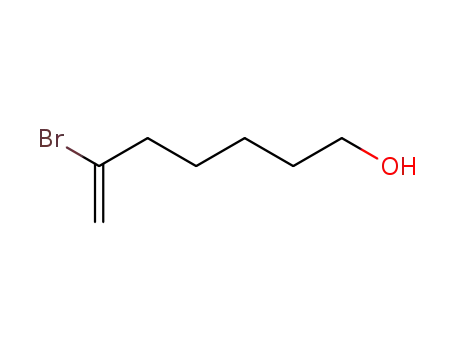
6-bromohept-6-en-1-ol
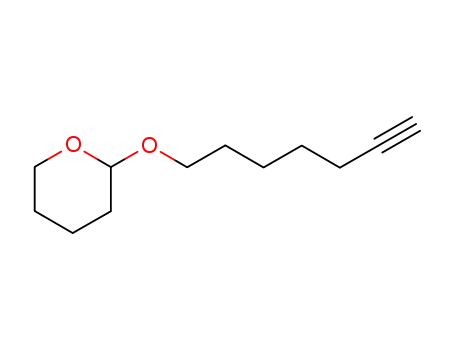
tetrahydro-2-(6-heptynyloxy)-2H-pyran
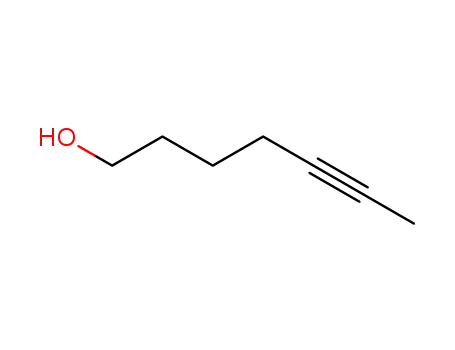
hept-5-yn-1-ol

tetrahydro-2-(6-heptynyloxy)-2H-pyran
2-<4-(6-heptyn-1-yloxy)phenyl>-4,5-dihydrooxazole

9-phenyl-8E-nonen-6-yn-1-ol

hept-5-yn-1-ol
CAS:2941-78-8
CAS:1143516-05-5
CAS:141-63-9
CAS:59-52-9