Your Location:Home >Products >Intermediates >100306-33-0
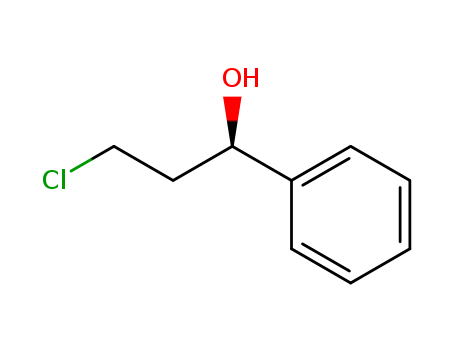

Product Details
|
Chemical Properties |
Off-white Cryst |
|
Uses |
Intermediate in the preparation of (S)-Norfluoxetine |
InChI:InChI=1/C9H11ClO/c10-7-6-9(11)8-4-2-1-3-5-8/h1-5,9,11H,6-7H2/t9-/m1/s1
Key residues of Debaryomyces hansenii ca...
A set of reaction conditions has been es...
Herein is presented a simple, attractive...
A new family of chiral iminophenyl oxazo...
New catalytic enantioselective reduction...
New spiroborate esters, derived from ter...
Penicillin G acylase from E. coli (E.C. ...
By enzyme screening, a ketoreductase clo...
Five endophytic yeast strains isolated f...
Biotransformation of 3-chloro-1-phenylpr...
Optically active 2-aryl substituted oxet...
Novel spiroborate esters derived nonrace...
Chiral secondary alcohols with additiona...
An efficient synthetic route to either R...
Enantioselective reduction of ketones wi...
A safe and inexpensive procedure for asy...
A variety of ketones can be reduced in h...
A diverse range of prochiral ketones wer...
A molecularly defined chiral boxmi iron ...
A new class of diamino diols was evaluat...
In this work, we report our results on t...
The invention discloses a preparation me...
Meayamycin B is currently the most poten...
The recently identified pseudoephedrine ...
Iridium-catalyzed asymmetric hydrogenati...
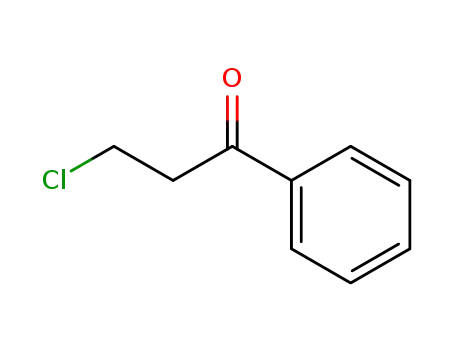
3-chloropropiophenone

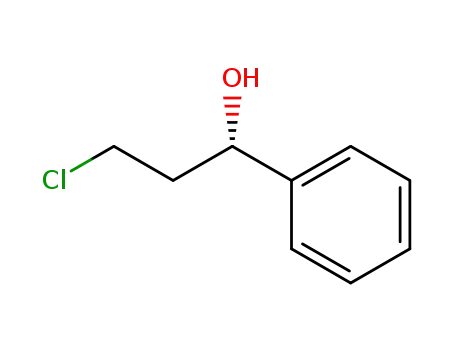
3-chloro-1-phenylpropanol

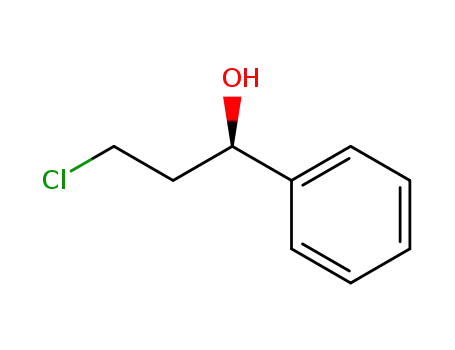
(1R)-3-chloro-1-phenylpropanol
| Conditions | Yield |
|---|---|
|
With dimethylsulfide borane complex; C23H22BNO3; In tetrahydrofuran; at 20 ℃; for 2h; Reagent/catalyst;
|
75% |
|
With dimethylsulfide borane complex; (+)-3-exo-amino-7,7-dimethoxynorbornan-2-exo-ol; In tetrahydrofuran; at 25 ℃; for 2h;
|
65% |
|
With dimethylsulfide borane complex; chiral diphenyloxazaborolidine; In tetrahydrofuran; at 25 ℃; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With lithium borohydride; N,N′-dibenzoyl-L-cysteine; tert-butyl alcohol; In tetrahydrofuran; at -78 - -30 ℃; for 9h; Yield given. Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With 9-borabicyclo[3.3.1]nonane dimer; borane-THF; (S)-diphenylprolinol; In tetrahydrofuran; at 20 ℃; for 1h; Title compound not separated from byproducts;
|
|
|
With borane; (2R,3S,4S,5R)-2,5-diamino-1,6-diphenyl-3,4-hexanediol; In tetrahydrofuran; at 35 ℃; for 5h; Title compound not separated from byproducts;
|
|
|
chiral benzodioxole-based copper; In tetrahydrofuran; toluene; tert-butyl alcohol; at -78 ℃; for 8h; Title compound not separated from byproducts;
|
|
|
With dimethylsulfide borane complex; In tetrahydrofuran; at 20 ℃; Title compound not separated from byproducts.;
|
|
|
With hydrogen; Cp*Ir(OTf)[(S,S)-Msdpen]; In methanol; at 60 ℃; for 24h; under 7600.51 Torr; Product distribution / selectivity;
|
77 % ee |
|
With ketoreductase 108; NADPH; at 30 ℃; pH=6.0; optical yield given as %ee; aq. phosphate buffer; Enzymatic reaction;
|
|
|
With borane N,N-diethylaniline complex; (S)-Corey-Bakshi-Shibata oxazaborolidine; In 1,2-dimethoxyethane; dichloromethane; water; at 25 - 30 ℃; for 12h; optical yield given as %ee; enantioselective reaction; Inert atmosphere;
|
|
|
With sodium t-butanolate; tert-butyl alcohol; (S)-2,2',6,6'-tetramethoxy-4,4'-bis(di(3,5-xylyl)phosphino)-3,3'-bipyridine; In toluene; at 0 ℃; for 14h; Overall yield = 90 %;
|
88 % ee |
|
With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; (S,S,S,S)-N,N-bis(1,2-diphenylethylenediamino)-1,3-benzenedisulfonylamine; sodium formate; In water; at 60 ℃; for 11h; enantioselective reaction; Inert atmosphere;
|
85.2 % ee |
|
With diborane; (S)-1-methyl-3,3-diphenyl-hexahydropyrrolo[1,2-c][1,3,2]oxazaborole;
|
82 % ee |
|
With dimethylsulfide borane complex; (1R,2S,3R,5R)-2-(1',3',2'-dioxaborolan-2'-yloxy)apopinan-3-amine; In tetrahydrofuran; at 20 ℃; for 1h; Overall yield = 96 %; enantioselective reaction;
|
80 % ee |
|
With diborane; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran; 2-methyltetrahydrofuran; at 0 ℃; Solvent; Temperature; Overall yield = 94 %; Overall yield = 92 mg; enantioselective reaction; Flow reactor; Green chemistry;
|
80 % ee |
|
With silver tetrafluoroborate; diethoxymethylane; C26H29N3O2*Cl(1-)*Ir(1+)*C8H12; at 20 ℃; for 20h; Overall yield = 82 %; stereoselective reaction;
|
66 % ee |
|
With D-glucose; dehydrogenase from Bacillus megaterium; ketoreductase cloned from Scheffersomyces stipitis CBS 6045; NADP; In aq. phosphate buffer; dimethyl sulfoxide; at 30 ℃; for 6h; pH=6.5; enantioselective reaction;
|
87.7 % ee |
|
With sodium tetrahydroborate; borane-THF; (R)-1,1'-Bi-2-naphthol; In tetrahydrofuran; at -78 - 24 ℃; for 12h; Overall yield = 49 percentSpectr.;
|

3-chloropropiophenone


(1R)-3-chloro-1-phenylpropanol
| Conditions | Yield |
|---|---|
|
With borane; S-oxaborolidine; In tetrahydrofuran; at 0 ℃; for 0.833333h;
|
99% |
|
With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; C22H32N4O4S2; water; sodium formate; at 40 ℃; for 0.25h; optical yield given as %ee; enantioselective reaction; Air atmosphere;
|
99% |
|
With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; (1R,2R)-9H-fluorene-2,7-disulfonic acid bis-[(2-amino-cyclohexyl)amide]; sodium formate; In water; at 40 ℃; for 0.25h; optical yield given as %ee; enantioselective reaction;
|
99% |
|
With C22H27Cl2CoN3O; sodium triethylborohydride; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane; In tetrahydrofuran; diethyl ether; at 20 ℃; for 2h; enantioselective reaction; Inert atmosphere;
|
97% |
|
With borane-THF; (S)-diphenylprolinol; In tetrahydrofuran; toluene; at 20 ℃; for 1.5h; enantioselective reaction;
|
97% |
|
With 2-[(1,3,2-dioxaborolan-2-yloxy)diphenylmethyl]pyrrolidine; dimethylsulfide borane complex; In tetrahydrofuran; at 20 ℃; for 2h;
|
95% |
|
With 2-[(1,3,2-dioxaborolan-2-yloxy)diphenylmethyl]pyrrolidine; dimethylsulfide borane complex; In tetrahydrofuran; at 20 ℃; for 2.16667h;
|
95% |
|
3-chloropropiophenone; With phenylsilane; copper(II) acetate monohydrate; (R)-(+)-2,2',6,6'-tetramethoxy-4,4'-bis(di(3,5-xylyl)phosphino)-3,3'-bipyridine; In toluene; at -20 ℃; for 48h;
With hydrogenchloride; In water; toluene; enantioselective reaction;
|
94% |
|
With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; C55H92N6O4S(2+)*2I(1-); sodium formate; In water; stereoselective reaction;
|
91% |
|
With sodium tetrahydroborate; Pinene; boron trichloride; In 1,2-dimethoxyethane; di-isopropyl ether; at -10 - 30 ℃; for 2h; Inert atmosphere;
|
91.8% |
|
With dimethylsulfide borane complex; 2-[(1,3,2-dioxaborolan-2-yloxy)diphenylmethyl]pyrrolidine; In tetrahydrofuran; for 2h; Product distribution / selectivity;
|
86% |
|
With (+)-diiso-2-ethylapophosphate pinacylboraneheptane; In tetrahydrofuran; at -20 ℃; for 6h; Temperature; stereoselective reaction;
|
86.7% |
|
With D-glucose; D-glucose dehydrogenase; Pichia guilliermondii; nicotinamide adenine dinucleotide phosphate; sodium hydroxide; In aq. phosphate buffer; dimethyl sulfoxide; at 20 ℃; for 6h; pH=6.5; Enzymatic reaction;
|
85% |
|
3-chloropropiophenone; With Triethoxysilane; (S,E)-(+)-2,6-diisopropyl-N-(2-((2-(4-phenyl-4,5-dihydrooxazol-2-yl)phenyl)amino)benzylidene)aniline; sodium triethylborohydride; cobalt(II) chloride; In tetrahydrofuran; dichloromethane; at 20 ℃; for 15h; Schlenk technique; Inert atmosphere;
With potassium carbonate; In tetrahydrofuran; methanol; dichloromethane; at 20 ℃; for 2h; enantioselective reaction; Schlenk technique; Inert atmosphere;
|
76% |
|
With hydrogen; Noyori's catalyst; In methanol; at 60 ℃; for 24h; under 7600.51 Torr; Product distribution / selectivity;
|
9% |
|
Multi-step reaction with 2 steps
1: NaBH4
2: 79 percent Chromat. / Sphingomonas paucimobilis NCIMB 8195 / dimethylformamide; H2O; various solvent(s) / 120 h
With sodium tetrahydroborate; Sphingomonas paucimobilis NCIMB 8195; In water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: 88 percent / NaBH4 / ethanol / 2 h / 20 °C
2: 93 percent / pyridine; DMAP / CH2Cl2 / 0 - 20 °C
3: aq. phosphate buffer; Novozyme 435 / 288 h / 30 °C / pH 7
With pyridine; dmap; sodium tetrahydroborate; phosphate buffer; novozyme 435; In ethanol; dichloromethane; 1: Reduction / 2: Esterification / 3: Hydrolysis;
|
|
|
Multi-step reaction with 3 steps
1: LiAlH4 / diethyl ether
2: pyridine / CH2Cl2
3: buffer pH 7 / lipase from Pseudomonas fluorescens (SAM-2)
With pyridine; lithium aluminium tetrahydride; buffer pH 7; In diethyl ether; dichloromethane;
|
|
|
With borane; (S)-1-methyl-3,3-diphenyl-hexahydropyrrolo[1,2-c][1,3,2]oxazaborole; In tetrahydrofuran;
|
|
|
With borane N,N-diethylaniline complex; (S)-Corey-Bakshi-Shibata oxazaborolidine; In 1,2-dimethoxyethane; dichloromethane; water; at 25 - 30 ℃; for 9h; optical yield given as %ee; enantioselective reaction; Inert atmosphere; Large scale reaction;
|
|
|
With D-glucose; lyophilized cells of Escherichia coli pET28a-CpAR2-BmGDH; potassium carbonate; In ethanol; at 30 ℃; pH=7; enantioselective reaction; Green chemistry;
|
|
|
With Debaryomyceshansenii carbonyl reductase N179S/I214F/S215G; NADPH; In aq. phosphate buffer; at 30 ℃; for 2h; pH=6.5; Reagent/catalyst; stereoselective reaction; Enzymatic reaction;
|
>99 % ee |
|
With bis(1,5-cyclooctadiene)diiridium(I) dichloride; C33H32FeNOP; hydrogen; potassium carbonate; In ethanol; hexane; at 25 - 30 ℃; for 12h; under 38002.6 Torr; enantioselective reaction; Inert atmosphere; Glovebox; Autoclave;
|
99 % ee |
|
Multi-step reaction with 2 steps
1: C32H33FeN3O2Si / toluene / 2 h / -40 - 20 °C / Schlenk technique; Glovebox
2: silica gel / toluene / 1 h / 20 °C / Schlenk technique; Glovebox
With C32H33FeN3O2Si; silica gel; In toluene;
|
|
|
With Arthrobacter sp. TS-15 recombinant ephedrine dehydrogenase; NADH; In aq. phosphate buffer; at 25 ℃; pH=7.5; enantioselective reaction; Green chemistry; Enzymatic reaction;
|
99 % ee |

3-chloropropiophenone
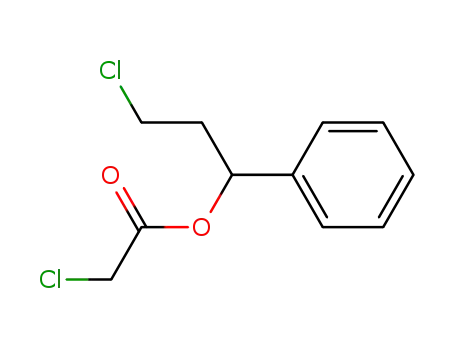
1-Phenyl-3-chlor-propyl-monochloracetat
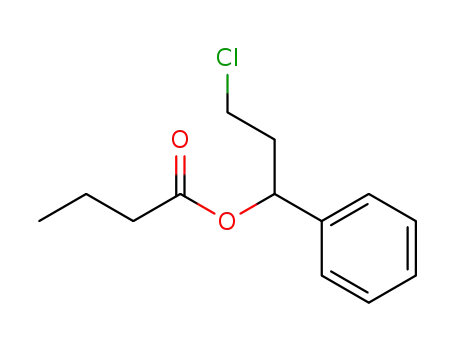
3-Cloro-1-phenylpropyl butanoate
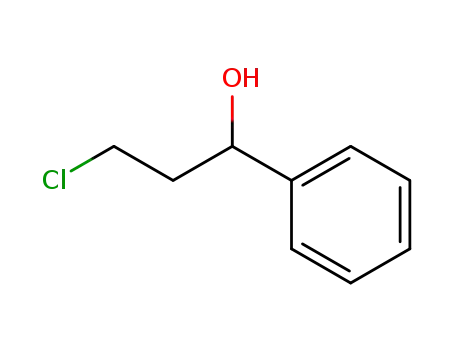
3-chloro-1-phenyl-propan-1-ol
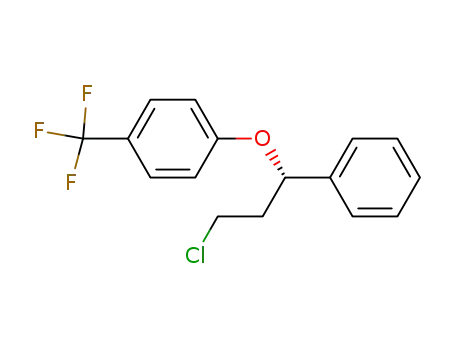
-(-)-1-chloro-3-phenyl-3-<4-(trifluoromethyl)phenoxy>propane
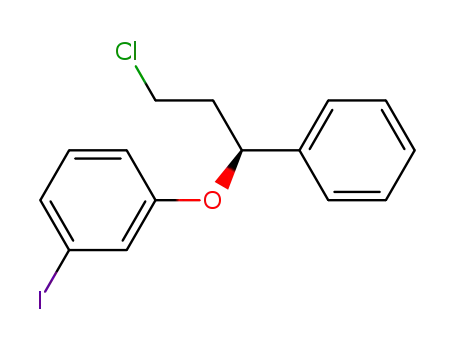
(S)-1-(3-chloro-1-phenylpropoxy)-3-iodobenzene
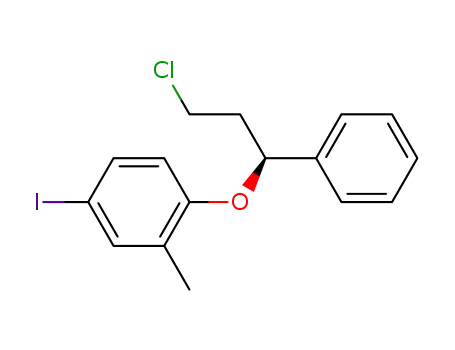
(S)-(-)-1-chloro-3-(4-iodo-2-methylphenoxy)-3-phenylpropane
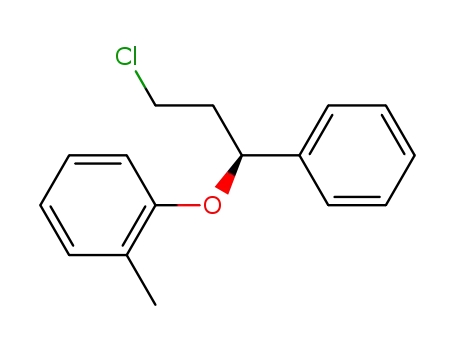
-(+)-1-chloro-3-phenyl-3-(2-methylphenoxy)propane
CAS:97-08-5
CAS:39065-95-7
CAS:51-74-1
CAS:95306-64-2