Your Location:Home >Products >Fine chemicals >4224-69-5
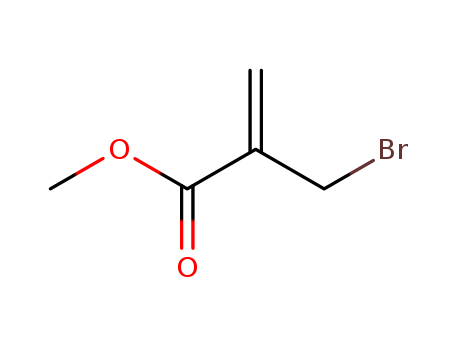

Product Details
|
Chemical Properties |
Clear colorless to slightly brownish liquid |
|
Synthesis Reference(s) |
Organic Syntheses, Coll. Vol. 7, p. 319, 1990Synthetic Communications, 16, p. 387, 1986 DOI: 10.1080/00397918608057713 |
InChI:InChI=1/C5H7BrO2/c1-4(3-6)5(7)8-2/h1,3H2,2H3
-
A novel protocol for the synthesis of te...
A radical-mediated approach to alkene ox...
The invention discloses a reaction type ...
The invention relates to chemically reac...
A novel strategy for the expedient const...
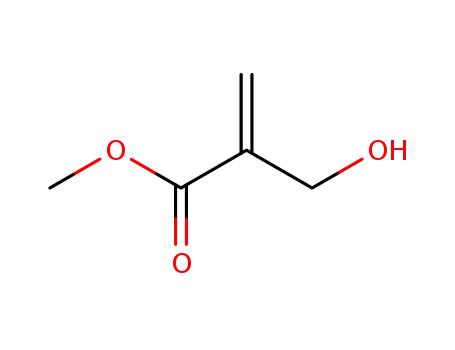
methyl 2-hydroxymethylacrylate

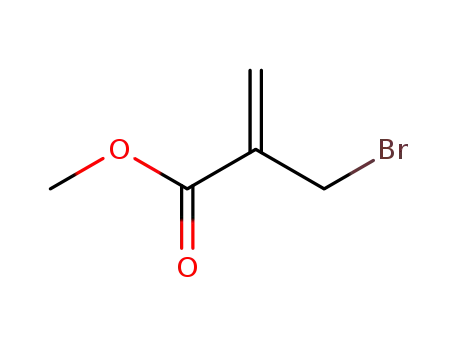
methyl 2-(bromomethyl)propenoate
| Conditions | Yield |
|---|---|
|
With phosphorus tribromide; In diethyl ether; Inert atmosphere;
|
89% |
|
With phosphorus tribromide; In diethyl ether; at 0 - 20 ℃; for 2h;
|
87.4% |
|
With phosphorus tribromide; In acetonitrile; at 20 ℃; for 4h;
|
86% |
|
With phosphorus tribromide; In diethyl ether; at 20 ℃; for 2h;
|
83% |
|
With phosphorus tribromide; In dichloromethane; at 0 - 20 ℃;
|
82% |
|
With hydrogen bromide; acetic acid; at 25 ℃; Inert atmosphere;
|
79% |
|
With hydrogen bromide; acetic acid; at 20 ℃; Inert atmosphere;
|
79% |
|
With sulfuric acid; hydrogen bromide;
|
76% |
|
With phosphorus tribromide; In dichloromethane; at 0 ℃; for 2.25h; Inert atmosphere;
|
72% |
|
With 1H-imidazole; bromine; triphenylphosphine; In dichloromethane; at 0 ℃; for 0.333333h;
|
68% |
|
With phosphorus tribromide; In diethyl ether; at -20 - 20 ℃; Inert atmosphere;
|
62% |
|
With phosphorus tribromide; In diethyl ether; for 3h; Yield given; Ambient temperature;
|
|
|
Multi-step reaction with 2 steps
1: during storage at low temperature
2: 76 percent / HBr, H2SO4
With sulfuric acid; hydrogen bromide;
|
|
|
With sulfuric acid; hydrogen bromide; at 0 ℃; for 0.166667h;
|
|
|
With sulfuric acid; hydrogen bromide;
|
|
|
With sulfuric acid; hydrogen bromide;
|
|
|
With phosphorus tribromide; In diethyl ether; at 0 ℃; for 1h; Inert atmosphere;
|
|
|
With phosphorus tribromide; In diethyl ether; at -5 ℃;
|
|
|
With sulfuric acid; hydrogen bromide; at 0 ℃; for 0.25h;
|
|
|
With phosphorus tribromide; In diethyl ether; at 20 ℃; for 3h; Inert atmosphere; Cooling;
|
|
|
With hydrogen bromide; acetic acid;
|
|
|
With phosphorus tribromide; In diethyl ether; at 20 ℃; for 3h; Cooling; Inert atmosphere;
|
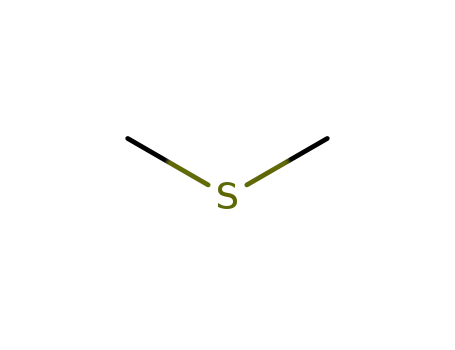
dimethylsulfide


methyl 2-hydroxymethylacrylate


methyl 2-(bromomethyl)propenoate
| Conditions | Yield |
|---|---|
|
With N-Bromosuccinimide; sodium chloride; In diethyl ether; dichloromethane;
|
89% |
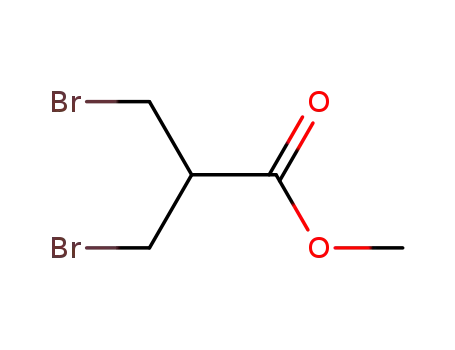
methyl 3-bromo-2-(bromomethyl)propionate

methyl 2-hydroxymethylacrylate
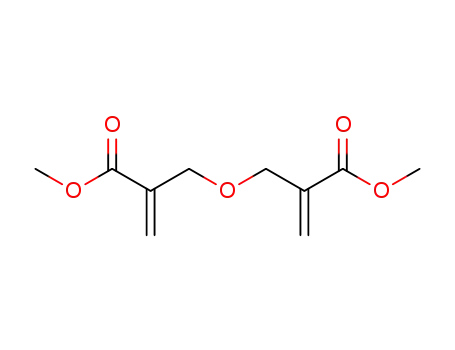
dimethyl 2,2′-[oxybis(methylene)]diacrylate
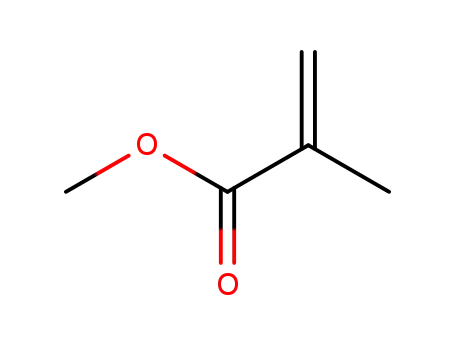
methacrylic acid methyl ester
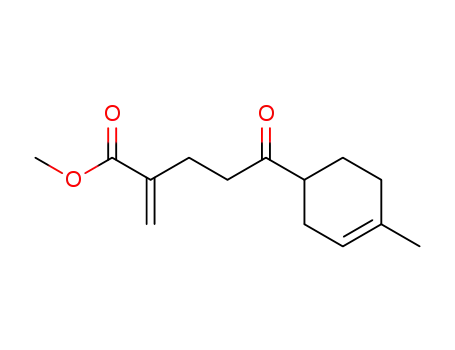
5-(4-Methyl-cyclohex-3-enyl)-2-methylene-5-oxo-pentanoic acid methyl ester
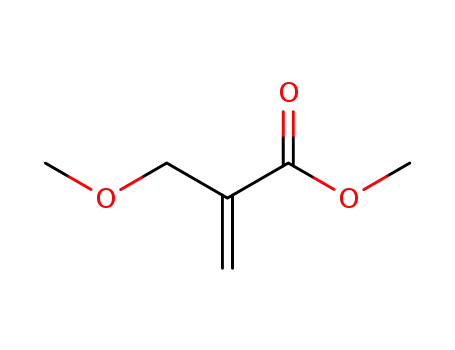
methyl α-methoxymethylacrylate
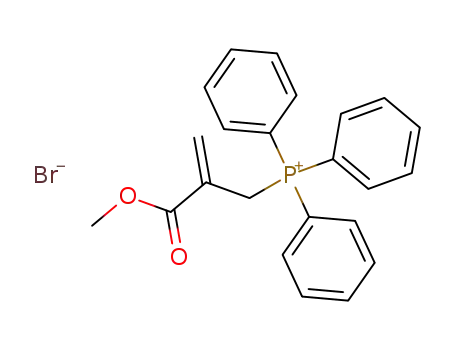
(2-(methoxycarbonyl)allyl)triphenylphosphonium bromide
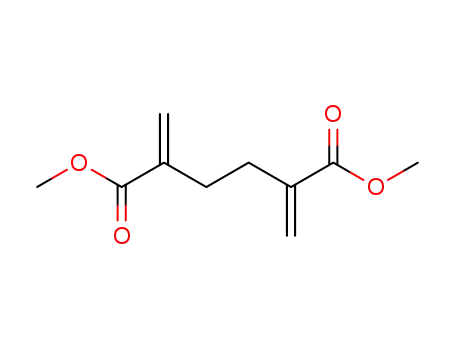
dimethyl 2,5-dimethylenehexanedioate
CAS:6482-24-2
CAS:114-49-8
CAS:1074-36-8
CAS:15174-69-3