Your Location:Home >Products >Fine chemicals >2424-92-2

Product Details
|
Uses |
Eicosanedioic Acid is an intermediate used to prepare haloperidol-based bivalent ligands targeting dopamine D2-like receptors. |
|
Definition |
ChEBI: An alpha,omega-dicarboxylic acid that is the 1,18-dicarboxy derivative of octadecane. |
InChI:InChI=1/C20H38O4/c21-19(22)17-15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16-18-20(23)24/h1-18H2,(H,21,22)(H,23,24)/p-2
-
The invention provides a method for obta...
The invention provides a method for prod...
The invention relates to a process for t...
Intramolecular end-to-end reactions of a...
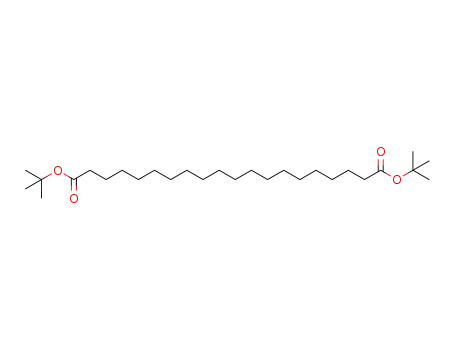
tert-butyl icosanedioic acid

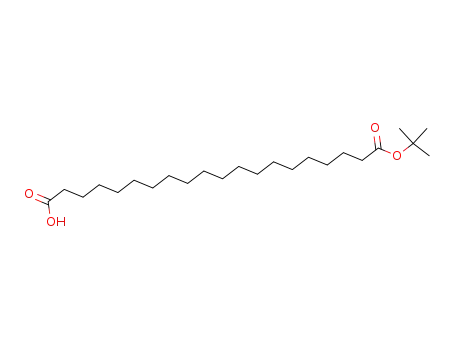
eicosanedioic acid mono(1,1-dimethylethyl)ester

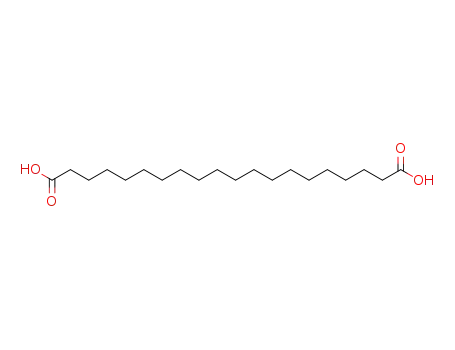
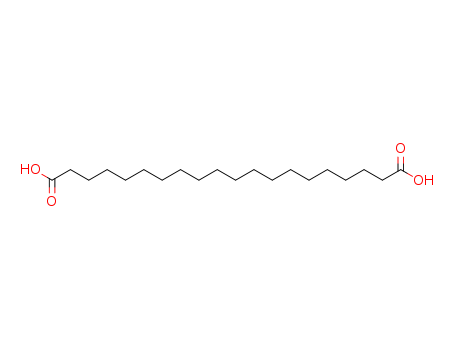
octadecane-1,18-dicarboxylic acid

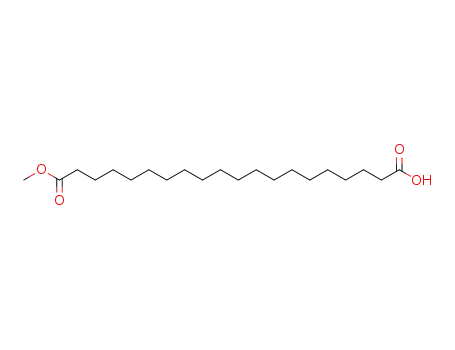
20-methoxy-20-oxoicosanoic acid
| Conditions | Yield |
|---|---|
|
With methanol; barium hydroxide octahydrate; at 30 - 35 ℃; for 14.5h;
|
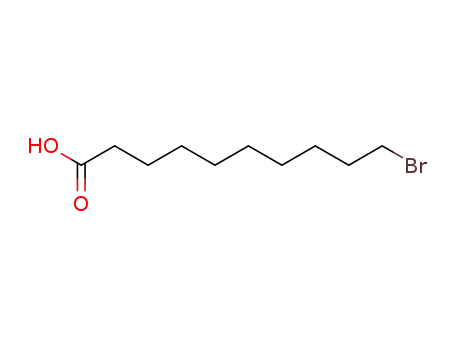
10-bromodecanoic acid


eicosanedioic acid mono(1,1-dimethylethyl)ester


octadecane-1,18-dicarboxylic acid


20-methoxy-20-oxoicosanoic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: trifluoroacetic anhydride / dichloromethane / 0.5 h / 0 °C / Large scale
1.2: 2 h / 0 - 10 °C / Large scale
2.1: zinc; nickel(II) chloride hexahydrate; 2,2':6,2''-terpyridine / N,N-dimethyl-formamide / 10 h / 35 - 45 °C / Inert atmosphere; Large scale
3.1: barium hydroxide octahydrate; methanol / 14.5 h / 30 - 35 °C
With methanol; 2,2':6,2''-terpyridine; nickel(II) chloride hexahydrate; barium hydroxide octahydrate; trifluoroacetic anhydride; zinc; In dichloromethane; N,N-dimethyl-formamide;
|
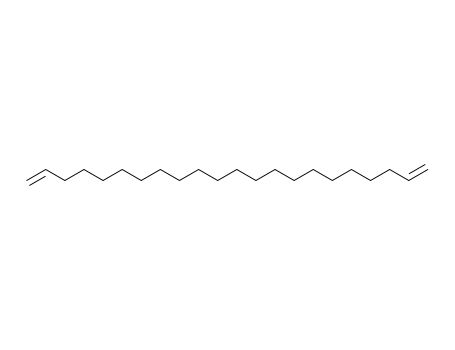
1,21-docosadiene
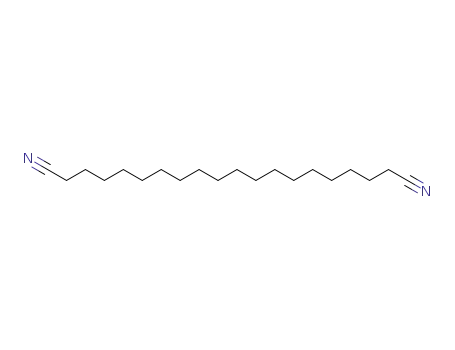
eicosanedinitrile
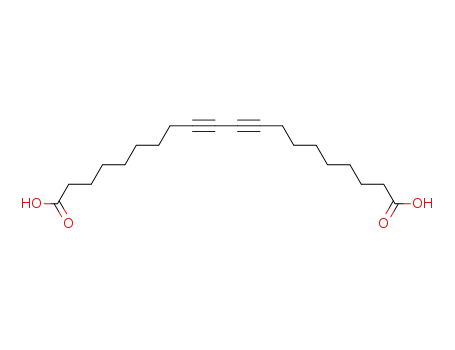
9,11-docosadiyn-1,20-dicarboxylic acid
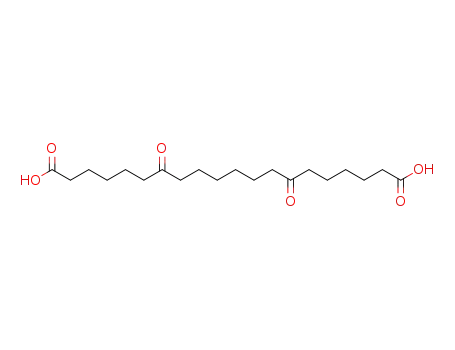
7,14-dioxo-eicosanedioic acid
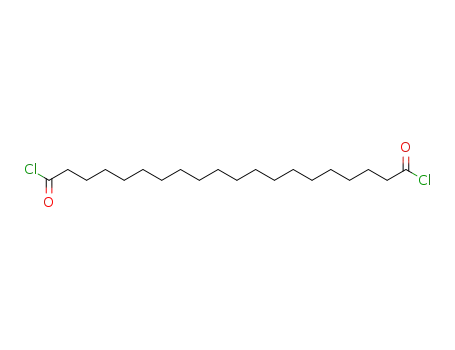
1,20-eicosanedioyl dichloride
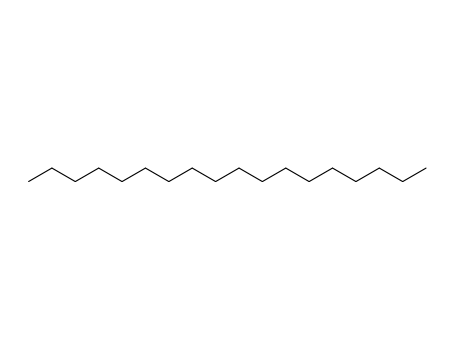
octadecane
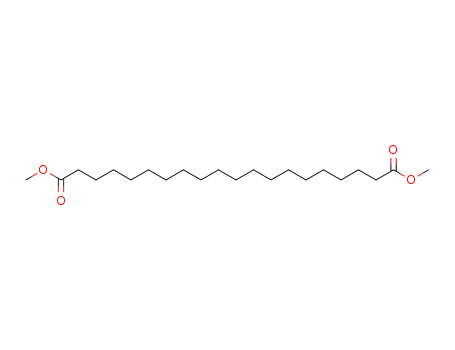
1,18-octadecanoic acid dimethyl ester
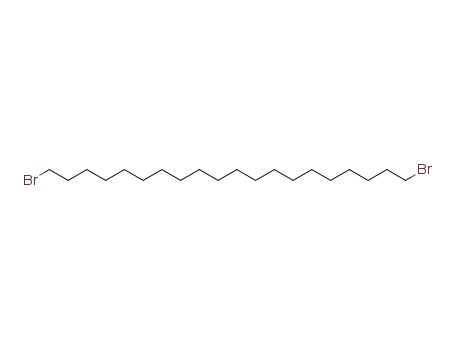
1,20-dibromoeicosane
CAS:2941-78-8
CAS:1143516-05-5
CAS:173772-63-9
CAS:615-62-3