Your Location:Home >Products >API >85622-93-1

Product Details
|
Description |
Temozolomide is a type of chemotherapy. It is also known as Temodal. It is a treatment for: certain types of brain tumour such as glioblastoma and astrocytoma. neuroendocrine tumours. |
|
Uses |
Temozolomide is used to treat specific types of brain cancer (eg, glioblastoma multiforme, anaplastic astrocytoma) in patients whose tumors have returned or whose tumors have just been diagnosed. It belongs to the group of medicines known as antineoplastics (cancer medicines). Take this medicine at the same time each day, either with or without food. Temozolomide often causes nausea and vomiting. However, it is very important that you continue to take the medicine, even if you begin to feel ill. You may take the medicine on an empty stomach or at bedtime may help to lessen the nausea. |
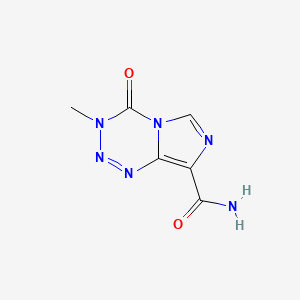
InChI:InChI=1/C6H6N6O2/c1-11-6(14)12-2-8-3(4(7)13)5(12)9-10-11/h2H,1H3,(H2,7,13)
Ethyl (8-carbamoyl-3,4-dihydro-4-oxoimid...
In this article the crystal structures o...
Temozolomide, an alkylating agent that can be administered orally, has been approved for the treatment of recurrent malignant glioma on a daily schedule for 5-day cycles. Continuous administration schedules with a higher dose intensity are being explored, but an improvement in efficiency remains to be shown.
This study created a new standard of adjuvant treatment, using concurrent and sequential temozolomide in the initial therapy of glioblastoma. Several preliminary studies have been initiated to address the issue of resistance and suppression of MGMT activity, and have used alternative temozolomide dosing schedules and O6-guanine mimetic agents as substrates for MGMT.
methyl isocyanate
5-diazoimidazole-4-carboxamide
(8-Carbamoyl-4-oxo-imidazo[5,1-d][1,2,3,5]tetrazin-3-yl)-acetic acid 2-thioxo-2H-pyridin-1-yl ester
dimethyl sulfate
<2H1>methan<2H>ol
dimethylphosphoric acid
deuteromethanol
CD2HOD
CAS:52865-10-8
CAS:1918-00-9
CAS:2941-78-8