Your Location:Home >Products >Intermediates >2941-78-8

Product Details
|
Chemical Properties |
Light-Brown Solid |
InChI:InChI=1/C8H9NO2/c1-5-2-3-7(9)6(4-5)8(10)11/h2-4H,9H2,1H3,(H,10,11)/p-1
Based on a multitarget strategy, a serie...
A Chiral Br?nsted acid catalyzed asymmet...
The invention discloses a synthetic meth...
Six stabilised phosphonium ylides bearin...
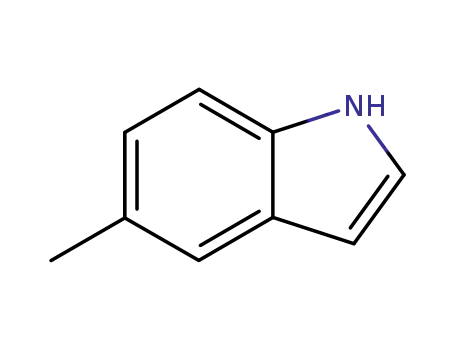
5-methyl-1H-indole

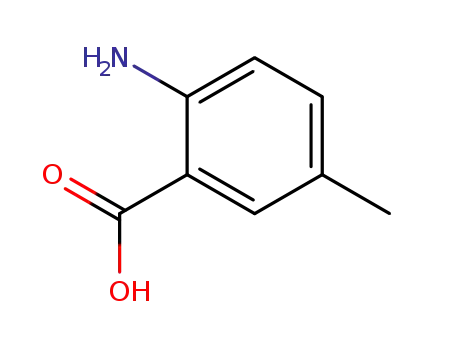
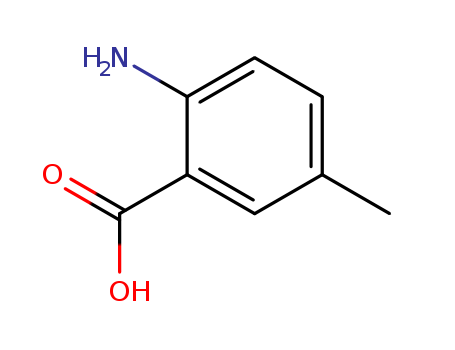
2-Amino-5-methylbenzoic acid
| Conditions | Yield |
|---|---|
|
5-methyl-1H-indole; With bromamine B; sodium hydroxide; palladium dichloride; In water; acetonitrile; at 60 ℃; for 3.33333h; pH=12;
In water; Acidic conditions;
|
96% |
|
With ruthenium trichloride; osmium(VIII) oxide; bromamine B; sodium hydroxide; In water; acetonitrile; at 39.84 ℃; for 4h;
|
96% |
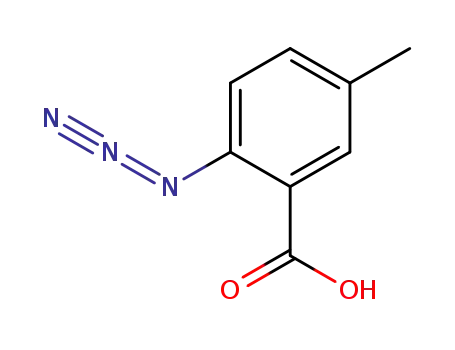
2-azido-5-methylbenzoic acid


2-Amino-5-methylbenzoic acid
| Conditions | Yield |
|---|---|
|
With boron trifluoride diethyl etherate; sodium iodide; In acetonitrile; at 20 ℃; for 0.666667h;
|
80% |
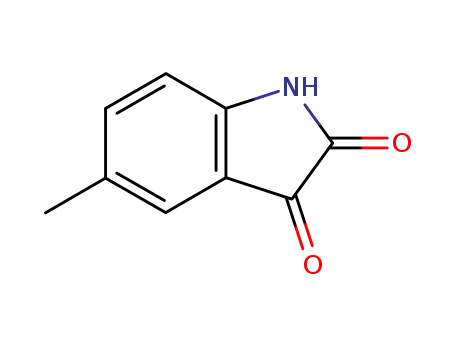
5-methyl-indole-2,3-dione
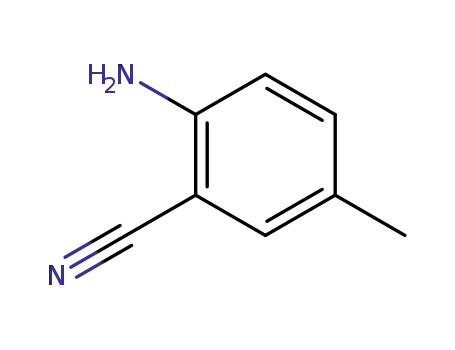
2-amino-5-methylbenzonitrile
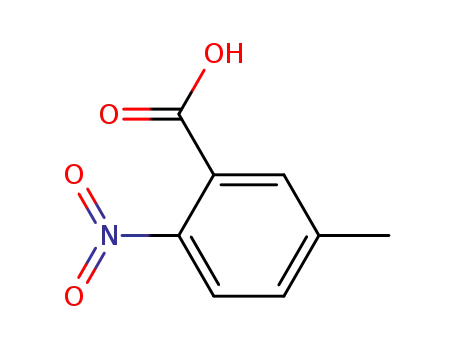
5-methyl-2-nitrobenzoic acid
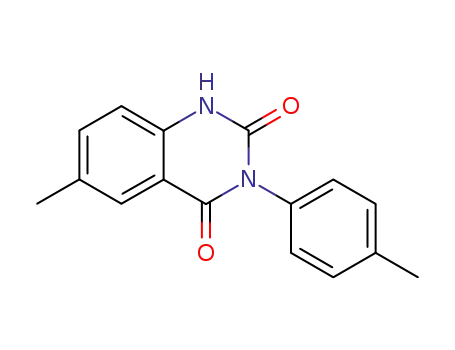
2,4-dioxo-3-(p-tolyl)-6-methyl-1,2,3,4-tetrahydroquinazoline
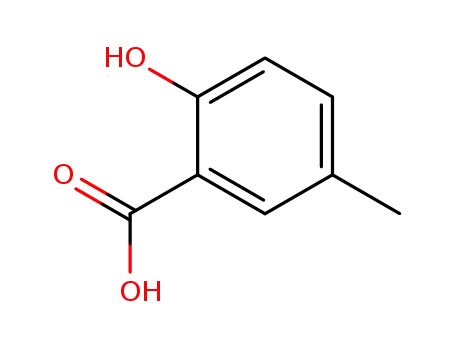
5-Methylsalicylic acid
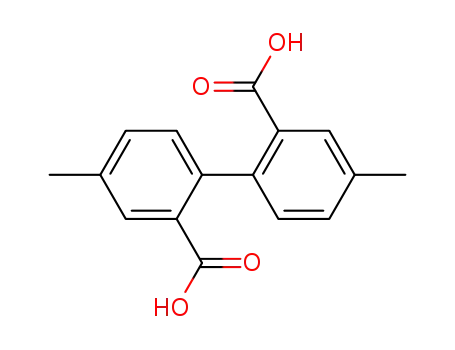
4,4'-dimethyl-diphenic acid
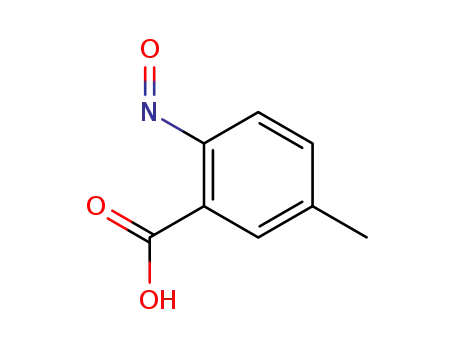
5-methyl-2-nitroso-benzoic acid
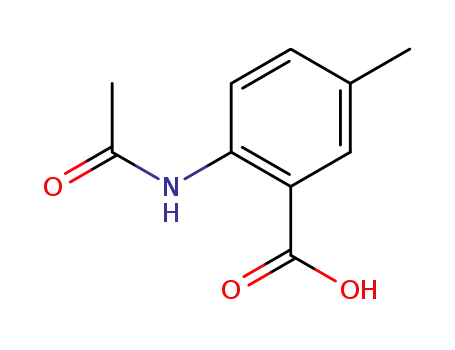
2-acetamido-5-methylbenzoic acid
CAS:1143516-05-5
CAS:882678-96-8
CAS:85622-93-1
CAS:89634-75-3