Your Location:Home >Products >Intermediates >882678-96-8
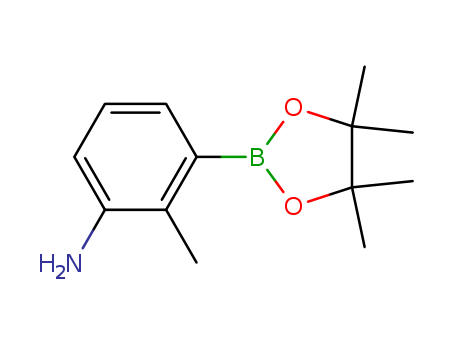

Product Details
|
General Description |
3-Amino-2-methylphenylboronic acid, pinacol ester is a chemical compound that belongs to the class of boronic acids. It is an ester derivative of 3-amino-2-methylphenylboronic acid, with the pinacol moiety serving as the ester group. 3-Amino-2-methylphenylboronic acid, pinacol ester is commonly used in organic synthesis as a reagent for the construction of carbon-carbon and carbon-heteroatom bonds. It is also utilized in the development of pharmaceuticals and agrochemicals. Additionally, its reactivity and versatility make it a valuable building block for the preparation of various complex molecules in the fields of medicinal chemistry and materials science. |
InChI:InChI=1/C13H20BNO2/c1-9-10(7-6-8-11(9)15)14-16-12(2,3)13(4,5)17-14/h6-8H,15H2,1-5H3
Herein, we report an efficient synthetic...
Immune checkpoint inhibitors targeting t...
The invention relates to a micromolecula...
Disclosed are a biaryl derivative having...
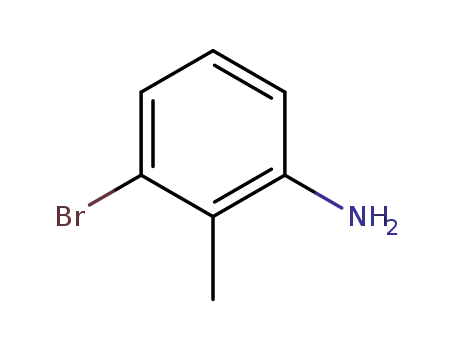
3-bromo-2-methylaniline

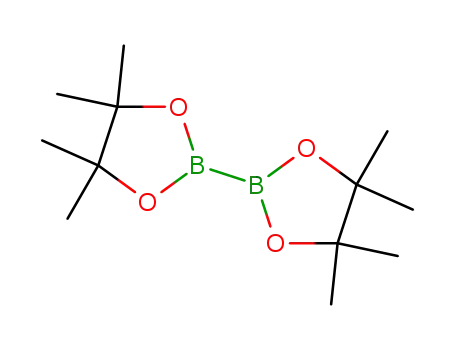
bis(pinacol)diborane

![2-methyl-3-( 4,4,5,5-tetramethyl[1,3,2]dioxaborolane-2-yl)phenylamine](/upload/2024/4/aff71947-a384-4eed-8d24-1404c825390e.png)
2-methyl-3-( 4,4,5,5-tetramethyl[1,3,2]dioxaborolane-2-yl)phenylamine
| Conditions | Yield |
|---|---|
|
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; In 1,4-dioxane; at 110 ℃; for 2.75h; Inert atmosphere;
|
91% |
|
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; In 1,4-dioxane; dimethyl sulfoxide; for 2h; Inert atmosphere; Reflux;
|
88% |
|
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; In 1,4-dioxane; dimethyl sulfoxide; for 2h; Reflux; Inert atmosphere;
|
88% |
|
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; In 1,4-dioxane; at 90 ℃; for 12h;
|
56.82% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In dimethyl sulfoxide; at 80 ℃; for 48h; Inert atmosphere;
|
39% |
|
With palladium bis[bis(diphenylphosphino)ferrocene] dichloride; potassium acetate; In dimethyl sulfoxide; at 80 ℃; for 22h;
|
39% |
|
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; at 120 ℃; for 2h;
|
|
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; at 80 ℃; for 16h; Inert atmosphere;
|
|
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane;
|
|
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; at 90 ℃; for 3h; Inert atmosphere;
|
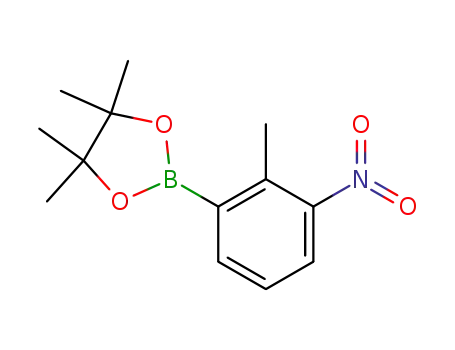
2-(2-methyl-3-nitrophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

![2-methyl-3-( 4,4,5,5-tetramethyl[1,3,2]dioxaborolane-2-yl)phenylamine](/upload/2024/4/aff71947-a384-4eed-8d24-1404c825390e.png)
2-methyl-3-( 4,4,5,5-tetramethyl[1,3,2]dioxaborolane-2-yl)phenylamine
| Conditions | Yield |
|---|---|
|
With hydrogen; palladium 10% on activated carbon; In methanol; at 20 ℃; for 13h;
|
100% |
|
With hydrogen; palladium 10% on activated carbon; In methanol; at 20 ℃; for 13h; Inert atmosphere;
|
100% |
|
With hydrogen; palladium 10% on activated carbon; In methanol; at 20 ℃; for 13h; Product distribution / selectivity;
|
100% |
|
With hydrogen; palladium on activated charcoal; In ethanol; at 20 ℃; for 6h;
|
|
|
With hydrogen; palladium 10% on activated carbon; In methanol; at 20 ℃; for 13h;
|
|
|
With palladium on activated charcoal; hydrogen; In ethanol; at 20 ℃; for 6h; Inert atmosphere;
|
3.12 g |
|
With platinum(IV) oxide; hydrogen; In tetrahydrofuran; at 20 ℃;
|
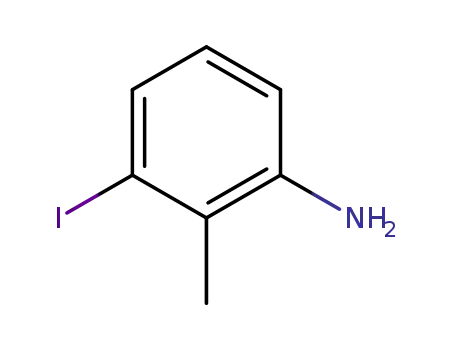
3-iodo-2-methylphenylamine

bis(pinacol)diborane

2-(2-methyl-3-nitrophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

3-bromo-2-methylaniline
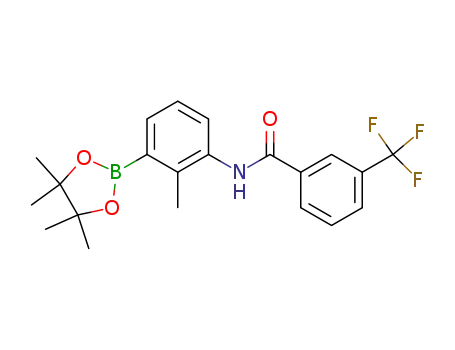
N-(2-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-3-(trifluoromethyl)benzamide
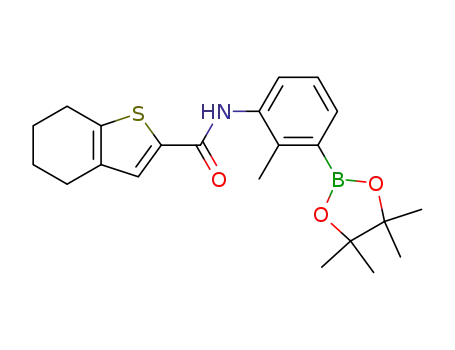
N-(2-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-4,5,6,7-tetrahydrobenzo[b]thiophene-2-carboxamide
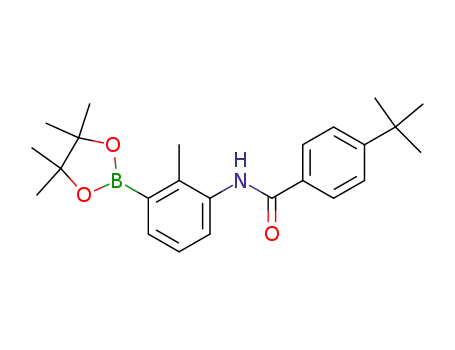
4-tert-butyl-N-[2-methyl-3-(4,4,5,5-tetramethyl-[1,2,3]dioxaborolan-2-yl)-phenyl]-benzamide
5-(3-amino-2-methylphenyl)-3-(4-(1,4-dimethyl-3-oxopiperazin-2-yl)phenylamino)-1-methylpyrazin-2(1h)-one
CAS:114-49-8
CAS:22536-67-0
CAS:14867-28-8