Your Location:Home >Products >Fine chemicals >14867-28-8
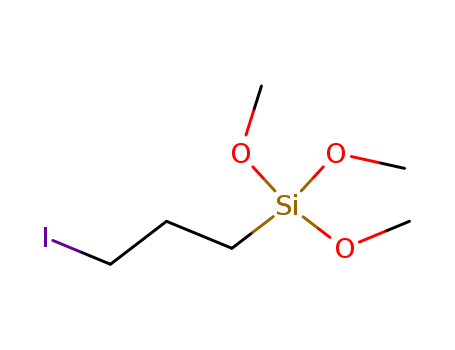

Product Details
|
Uses |
(3-iodopropyl)trimethoxysilane may be used to organically modify mesoporous silica support by grafting method.{85} The product may be used to synthesize heavy atom-concentrated?organically modified silica?nanoparticles.{86} |
InChI:InChI=1/C6H15IO3Si/c1-8-11(9-2,10-3)6-4-5-7/h4-6H2,1-3H3
We report on the catalytic activity for ...
A first example of simultaneous covalent...
Methods have been developed for the prep...
To investigate the original and promisin...
Silica nanoparticles linked through ioni...
Core-shell hybrid particles, possessing ...
A silica-supported iron complex has been...
A magnetically robust recyclable nanocat...
In this study, we report recyclable Pd-c...
A novel hybrid magnetic nanocatalyst was...
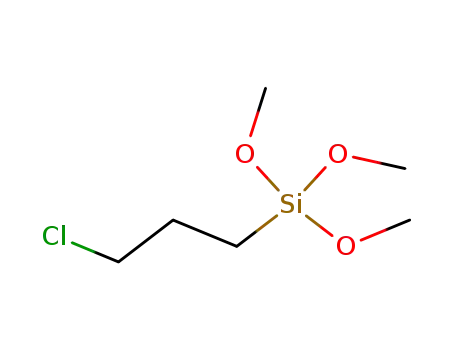
3-Chloropropyltrimethoxysilan

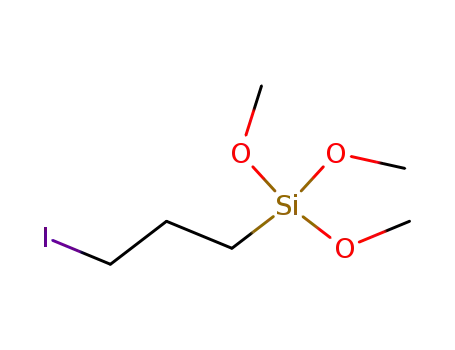
3-iodopropyltrimethoxysilane
| Conditions | Yield |
|---|---|
|
With sodium iodide; In acetone; Inert atmosphere; Reflux;
|
96% |
|
With sodium iodide; In acetone; for 24h; Inert atmosphere; Reflux;
|
90% |
|
With sodium iodide; In acetone; Reflux; Inert atmosphere;
|
90% |
|
With sodium iodide; In acetone; for 24h; Heating;
|
85% |
|
With sodium iodide;
|
|
|
With sodium iodide; In acetone; for 24h; Heating;
|
|
|
With sodium iodide; In acetone; Heating;
|
|
|
With sodium iodide; In acetone; Inert atmosphere; Heating;
|
|
|
With sodium iodide; In acetone; Inert atmosphere; Reflux;
|
|
|
With sodium iodide; In acetone; at 50 ℃; for 48h; Inert atmosphere;
|
|
|
With sodium iodide; In acetone; for 24h; Reflux;
|
|
|
With sodium iodide; In acetone; Heating; Inert atmosphere;
|
|
|
With sodium iodide; In acetone; at 80 ℃; Inert atmosphere;
|
|
|
With sodium iodide; In acetone; for 24h; Reflux; Inert atmosphere;
|
|
|
With potassium iodide; In acetone; for 12h; Reflux;
|


3-iodopropyltrimethoxysilane
| Conditions | Yield |
|---|---|
|
Cl(CH2)3Si(OCH3)3, NaI, in Acn., Kochen, 2 h;
|

3-Chloropropyltrimethoxysilan
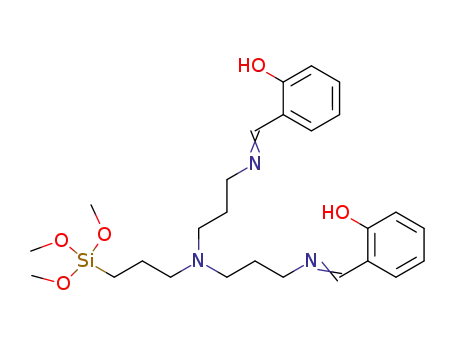
(CH3O)3SiC3H6N(C3H6NCHC6H4OH)2
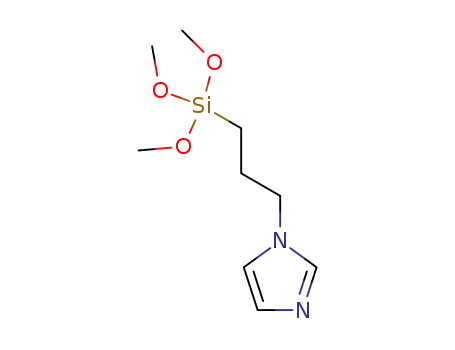
1-[3-(trimethoxysilyl)propyl]imidazole
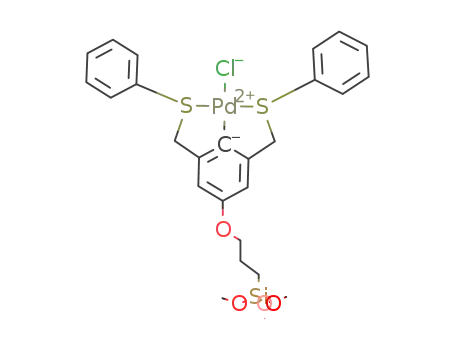
Pd-Cl 3-((3,5-bis(phenylsulfanylmethyl)phenoxy)propyltrimethoxysilane
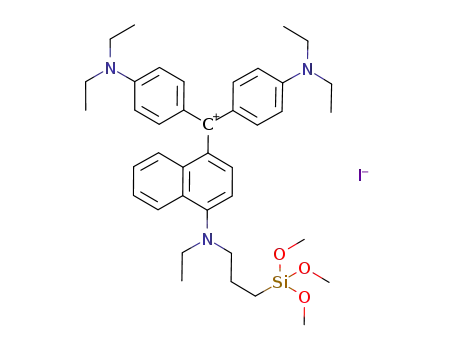
C39H54N3O3Si(1+)*I(1-)
CAS:96797-15-8
CAS:882678-96-8