Your Location:Home >Products >Intermediates >877399-73-0

Product Details
|
General Description |
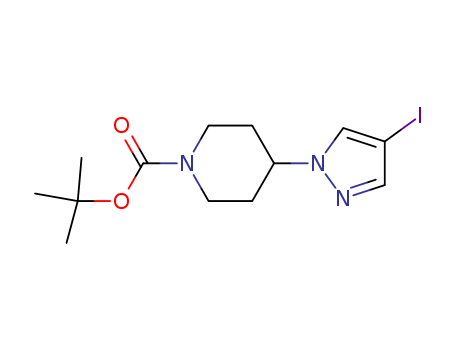
1-Piperidinecarboxylic acid, 4-(4-iodo-1H-pyrazol-1-yl)-, 1,1-dimethylethyl ester, also known as Tert-butyl 4-(4-iodo-1H-pyrazol-1-yl)piperidine-1-carboxylate, is a chemical compound used in organic synthesis and pharmaceutical research. It is commonly employed as a building block in the development of new drugs and agrochemicals. This ester derivative is known for its potential therapeutic activities and is used as a starting material in the production of various pharmaceuticals and biologically active compounds. Its unique chemical structure and properties make it a valuable component in drug discovery and development efforts. |
InChI:InChI=1/C13H20IN3O2/c1-13(2,3)19-12(18)16-6-4-11(5-7-16)17-9-10(14)8-15-17/h8-9,11H,4-7H2,1-3H3
The invention relates to a piperidine-co...
The invention belongs to the field of ch...
The present disclosure is directed to co...
The invention discloses a preparation me...
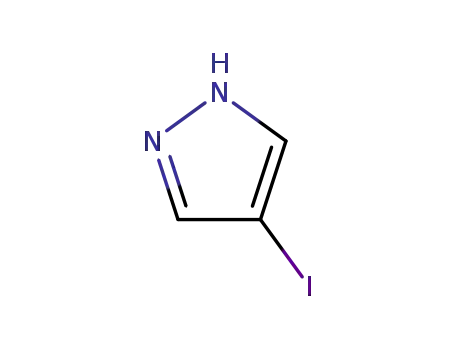
4-iodopyrazole

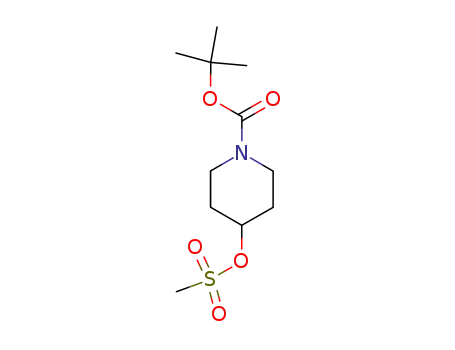
1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate

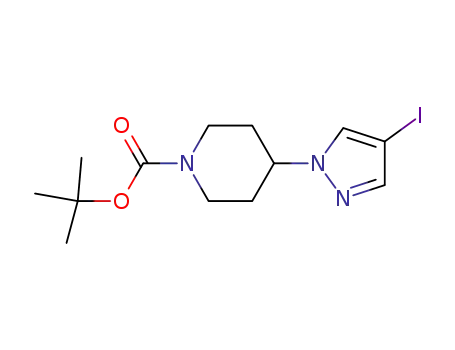
tert-butyl 4-(4-iodo-1H-pyrazol-1-yl)piperidine-1-carboxylate
| Conditions | Yield |
|---|---|
|
4-iodopyrazole; With sodium hydride; In dichloromethane; mineral oil; at 0 ℃; for 1h;
1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate; In dichloromethane; mineral oil; at 100 ℃; for 15h;
|
70.2% |
|
4-iodopyrazole; With sodium hydride; In dichloromethane; mineral oil; at 0 ℃; for 1h;
1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate; In dichloromethane; mineral oil; at 100 ℃; for 15h;
|
70.2% |
|
With sodium hydride; In N,N-dimethyl-formamide; at 100 ℃; for 14h; Cooling with ice;
|
68.7% |
|
With NaH; In N,N-dimethyl-formamide; pentane;
|
66% |
|
4-iodopyrazole; With sodium hydride; In N,N-dimethyl-formamide; at 4 ℃; for 1h;
1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate; In N,N-dimethyl-formamide; at 100 ℃; for 12h;
|
66% |
|
4-iodopyrazole; With sodium hydride; In N,N-dimethyl-formamide; at 4 ℃; for 1h;
1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate; In 1,2-dimethoxyethane; at 100 ℃; for 12h;
|
66% |
|
4-iodopyrazole; With sodium hydride; In N,N-dimethyl-formamide; at 4 ℃; for 1h;
1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate; In N,N-dimethyl-formamide; at 100 ℃; for 12h;
|
66% |
|
4-iodopyrazole; With sodium hydride; In N,N-dimethyl-formamide; at 4 ℃; for 1h; Inert atmosphere;
1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate; In N,N-dimethyl-formamide; at 100 ℃; for 12h; Inert atmosphere;
|
66% |
|
4-iodopyrazole; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 4 ℃; for 1h;
1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate; In N,N-dimethyl-formamide; mineral oil; at 100 ℃; for 12h;
|
66% |
|
4-iodopyrazole; With sodium hydride; In N,N-dimethyl-formamide; at 4 ℃; for 1h;
1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate; In N,N-dimethyl-formamide; at 4 - 100 ℃; for 12h;
|
66% |
|
4-iodopyrazole; With sodium hydride; In N,N-dimethyl-formamide; at 4 ℃; for 1h;
1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate; In N,N-dimethyl-formamide; at 100 ℃; for 12h;
|
66% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 60 ℃;
|
60.67% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 60 ℃;
|
60.67% |
|
With caesium carbonate; In n-methyl-2-pyrrilidone; at 80 ℃; for 18.5h;
|
57% |
|
With caesium carbonate; at 80 ℃; for 8h; Inert atmosphere;
|
57.9% |
|
at 80 ℃; for 8h; Inert atmosphere;
|
57.9% |
|
With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 110 ℃; for 0.5h; Inert atmosphere; Microwave irradiation;
|
55% |
|
4-iodopyrazole; 1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; Inert atmosphere;
In N,N-dimethyl-formamide; mineral oil; at 110 ℃; for 0.5h; Microwave irradiation; Inert atmosphere;
|
55% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 100 ℃;
|
51% |
|
4-iodopyrazole; With sodium hydride; In N,N-dimethyl-formamide; mineral oil; at 0 ℃; for 1h;
1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate; In N,N-dimethyl-formamide; mineral oil; at 100 ℃; for 16h;
|
41% |
|
4-iodopyrazole; With sodium hydride; In N,N-dimethyl-formamide; at 0 - 20 ℃; for 2h;
1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate; at 100 ℃; for 12h;
|
620 mg |
|
With sodium hydride; In N,N-dimethyl-formamide;
|
|
|
With caesium carbonate; In 1-methyl-pyrrolidin-2-one; at 70 ℃; for 5h; Temperature;
|
|
|
4-iodopyrazole; With sodium hydride; In N,N-dimethyl-formamide; at 0 ℃; for 1h;
1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate; In N,N-dimethyl-formamide; at 100 ℃; for 12h; Enzymatic reaction;
|
|
|
With caesium carbonate; In 1-methyl-pyrrolidin-2-one; at 80 ℃; for 16h;
|
900 mg |

4-iodopyrazole

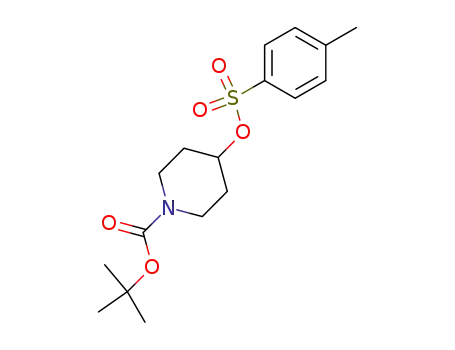
tert-butyl 4-(tosyloxy)piperidin-1-carboxylate


tert-butyl 4-(4-iodo-1H-pyrazol-1-yl)piperidine-1-carboxylate
| Conditions | Yield |
|---|---|
|
With caesium carbonate; In N,N-dimethyl acetamide; at 100 ℃; for 1h; Inert atmosphere;
|
92% |
|
With caesium carbonate; In N,N-dimethyl acetamide; at 100 ℃; for 1h; Inert atmosphere;
|
92% |

4-iodopyrazole

1-(tert-butoxycarbonyl)piperidin-4-yl methanesulfonate
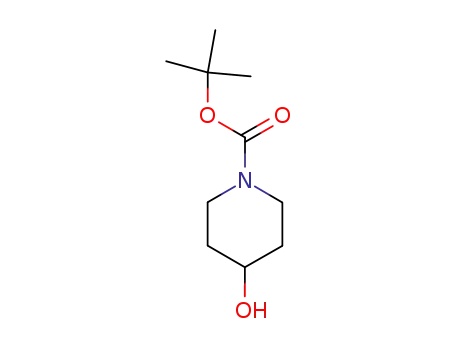
t-butyl 4-hydroxy piperidine-1-carboxylate
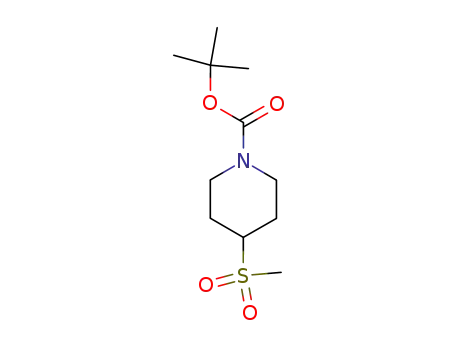
4-methanesulfonylpiperidine-1-carboxylic acid tert-butyl ester
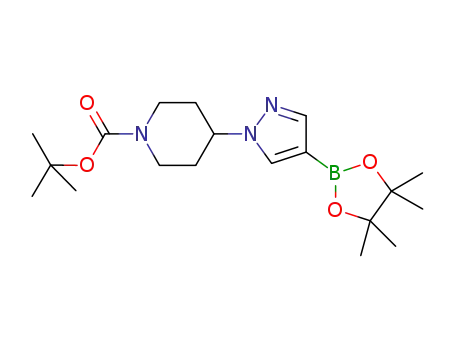
tert-butyl 4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)piperidine-1-carboxylate
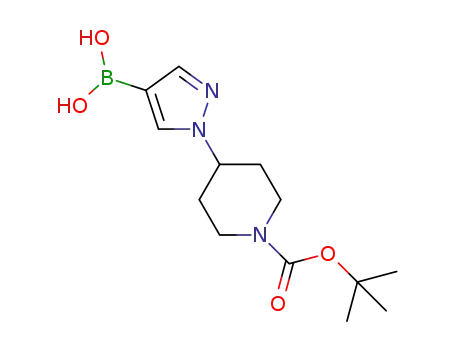
(1-{1-[(tert-butoxy)carbonyl]piperidin-4-yl}-1H-pyrazol-4-yl)boronic acid
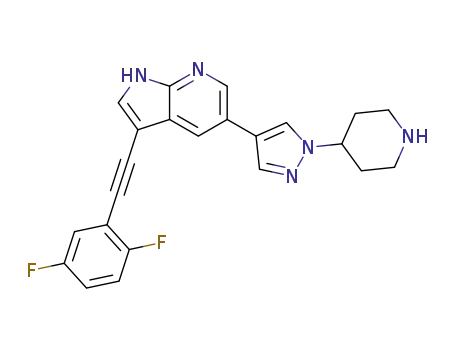
3-((2,5-difluorophenyl)ethynyl)-5-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)-1H-pyrrolo[2,3-b]pyridine
CAS:96797-15-8
CAS:14867-28-8
CAS:13360-57-1
CAS:30827-99-7