Your Location:Home >Products >Intermediates >96797-15-8
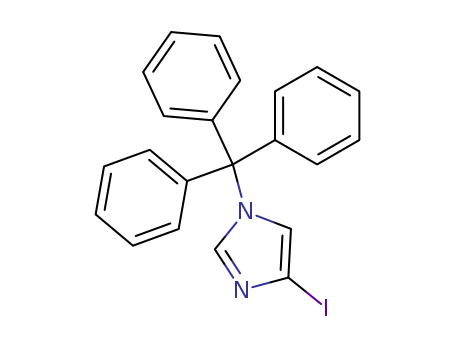

Product Details
|
Uses |
4-Iodo-1-tritylimidazole is a compound useful in organic synthesis. |
|
Chemical Properties |
White Solid |
InChI:InChI=1/C22H17IN2/c23-21-16-25(17-24-21)22(18-10-4-1-5-11-18,19-12-6-2-7-13-19)20-14-8-3-9-15-20/h1-17H
The invention belongs to the technical f...
The present application belongs to the f...
Indoleamine 2,3-dioxygenase (IDO) 1 is t...
The present invention relates to O-GIcNA...
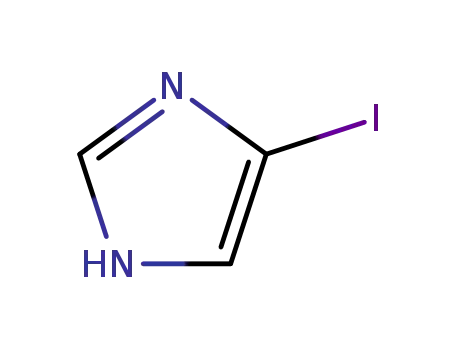
4-iodoimidazole

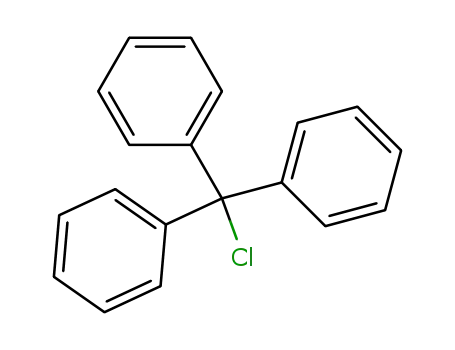
trityl chloride

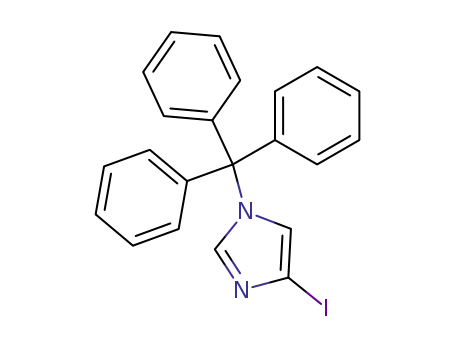
N-trityl-4(5)-iodoimidazole
| Conditions | Yield |
|---|---|
|
With triethylamine; In N,N-dimethyl-formamide; at 20 - 25 ℃; for 22h; Solvent; Reagent/catalyst; Temperature;
|
97.2% |
|
With triethylamine; In N,N-dimethyl-formamide; Ambient temperature;
|
95% |
|
With triethylamine; In N,N-dimethyl-formamide; at 20 ℃; for 24h;
|
94% |
|
With triethylamine; In N,N-dimethyl-formamide; at 20 ℃; for 24h;
|
94% |
|
With triethylamine; In DMF (N,N-dimethyl-formamide); at 20 ℃; for 48h;
|
93% |
|
With triethylamine; In DMF (N,N-dimethyl-formamide); at 20 ℃; for 48h;
|
93% |
|
With triethylamine; In dichloromethane; at 25 ℃; for 17h; Cooling with ice; Inert atmosphere;
|
93% |
|
With triethylamine; In dichloromethane; at 25 ℃; for 17h; Inert atmosphere;
|
93% |
|
With triethylamine; In dichloromethane; at 25 ℃; for 17h; Inert atmosphere;
|
93% |
|
In DMF (N,N-dimethyl-formamide); at 20 ℃; for 24h;
|
92% |
|
4-iodoimidazole; With triethylamine; In N,N-dimethyl-formamide; at 0 ℃; for 0.166667h;
trityl chloride; In N,N-dimethyl-formamide; at 20 ℃; for 16h;
|
92% |
|
In ISOPROPYLAMIDE; at 0 - 20 ℃;
|
90% |
|
With triethylamine; In N,N-dimethyl-formamide; at 20 ℃; for 48h;
|
88.9% |
|
With triethylamine; In dichloromethane; at 20 ℃; for 15h;
|
88% |
|
With triethylamine; In tetrahydrofuran; at 70 ℃; for 3h; Inert atmosphere;
|
81% |
|
With N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 20 ℃;
|
78% |
|
With triethylamine; In N,N-dimethyl-formamide; Ambient temperature;
|
76% |
|
With triethylamine; In tetrahydrofuran; at 80 ℃;
|
71% |
|
With triethylamine; In N,N-dimethyl-formamide; at 20 ℃; for 14h;
|
66% |
|
With triethylamine; In tetrahydrofuran; for 1.5h; Reflux;
|
64% |
|
With triethylamine; In water; N,N-dimethyl-formamide;
|
59% |
|
4-iodoimidazole; With triethylamine; In dichloromethane; at 20 ℃; for 0.0833333h;
trityl chloride; In dichloromethane; at 40 ℃; for 16h;
|
58% |
|
With triethylamine; In tetrahydrofuran; at 70 ℃; for 3h;
|
55.57% |
|
With triethylamine; In tetrahydrofuran;
|
|
|
|
|
|
With triethylamine; In dichloromethane;
|
|
|
With triethylamine; In N,N-dimethyl-formamide;
|
|
|
With triethylamine; In N,N-dimethyl-formamide; at 20 ℃; for 16h;
|

4-iodoimidazole

diethyl ether


trityl chloride


N-trityl-4(5)-iodoimidazole
| Conditions | Yield |
|---|---|
|
In N,N-dimethyl-formamide;
|
92% |

4-iodoimidazole

trityl chloride

diethyl ether
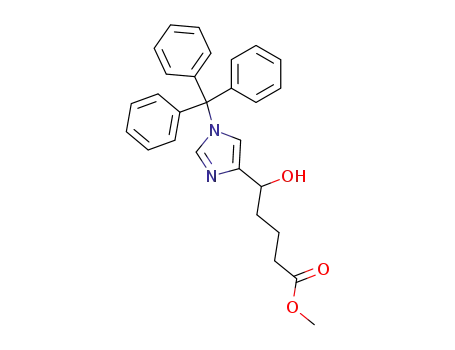
5-Hydroxy-5-(1-trityl-1H-imidazol-4-yl)-pentanoic acid methyl ester
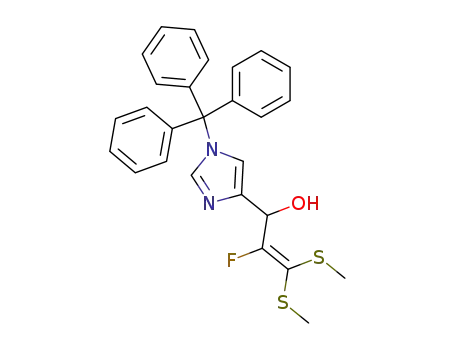
1,1-Bis(methylthio)-2-fluoro-3-((1'-trityl)-4'-imidazolyl)prop-1-en-3-ol
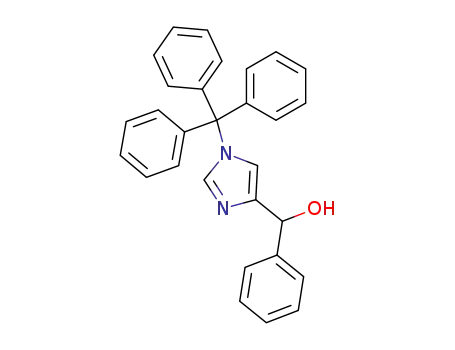
1-triphenylmethyl-4-[(1-hydroxy-1-phenyl)methyl]-1H-imidazole
alpha-methyl-1-triphenylmethyl-1H-imidazole-4-methanol
CAS:14867-28-8
CAS:18513-76-3
CAS:936-59-4