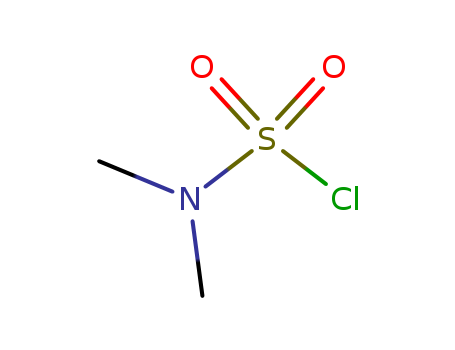
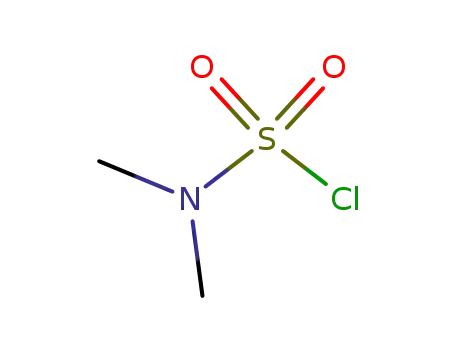
Factory Supply High Purity Dimethylsulfamoyl chloride 13360-57-1 with Low Price
- Molecular Formula:C2H6ClNO2S
- Molecular Weight:143.594
- Appearance/Colour:clear colorless to slightly yellow liquid
- Vapor Pressure:0.653mmHg at 25°C
- Melting Point:-13 °C
- Refractive Index:n20/D 1.452(lit.)
- Boiling Point:186.7 °C at 760 mmHg
- PKA:-8.25±0.70(Predicted)
- Flash Point:94.4 °C
- PSA:45.76000
- Density:1.418 g/cm3
- LogP:1.11240
Dimethylsulfamoyl chloride(Cas 13360-57-1) Usage
|
Chemical Properties
|
clear colorless to slightly yellow liquid
|
|
Uses
|
Dichlorofluanid is a degradation product of the pesticide Dichlorofluanid.
|
InChI:InChI=1/C2H6ClNO2S/c1-4(2)7(3,5)6/h1-2H3
13360-57-1 Relevant articles
-
Schneider et al.
, p. 239,240, 242, 243 (1968)
-
-
Tanaka et al.
, p. 1324 (1978)
-
-
Binkley,Degering
, p. 3250 (1939)
-
Semicarbazone-based inhibitors of cathepsin K, are they prodrugs for aldehyde inhibitors?
Adkison, Kim K.,Barrett, David G.,Deaton, David N.,Gampe, Robert T.,Hassell, Anne M.,Long, Stacey T.,McFadyen, Robert B.,Miller, Aaron B.,Miller, Larry R.,Payne, J. Alan,Shewchuk, Lisa M.,Wells-Knecht, Kevin J.,Willard Jr., Derril H.,Wright, Lois L.
, p. 978 - 983 (2007/10/03)
Starting from potent aldehyde inhibitors...
6,6-BICYCLIC RING SUBSTITUTED HETEROBICYCLIC PROTEIN KINASE INHIBITORS
-
Page/Page column 392-395, (2008/06/13)
Compounds of the formula (I) and pharmac...
Potent and selective, sulfamide-based human β3-adrenergic receptor agonists
Dow, Robert L.,Paight, Ernest S.,Schneider, Steven R.,Hadcock, John R.,Hargrove, Diane M.,Martin, Kelly A.,Maurer, Tristan S.,Nardone, Nancy A.,Tess, David A.,DaSilva-Jardine, Paul
, p. 3235 - 3240 (2007/10/03)
A series of sulfamide-based analogs rela...
Synthesis and structure of 2-N,N-dimethyl- and 2-N,N-diethylsulfamoyl-1-(3-indolyl)-1,2-dihydroisoquinolines
Skrypnik,Vasil'eva,Lyashchuk,Bezrodnyi,Enya
, p. 577 - 580 (2007/10/03)
New sulfamoyl derivatives of (3-indolyl)...
13360-57-1 Process route
-
- 506-59-2
N,N-dimethylammonium chloride
-
- 13360-57-1
dimethylamino sulfonyl chloride
Conditions
| Conditions |
Yield |
|
With sulfuryl dichloride; for 12h; Heating;
|
70% |
|
With sulfuryl dichloride;
|
|
|
With sulfuryl dichloride;
|
|
-
- 13360-57-1
dimethylamino sulfonyl chloride
Conditions
| Conditions |
Yield |
|
With sulfuryl dichloride;
|
|
|
With sulfuryl dichloride; triethylamine; In 1,4-dioxane; chloroform; at 0 - 20 ℃;
|
|
13360-57-1 Upstream products
-
506-59-2
N,N-dimethylammonium chloride
-
124-40-3
dimethyl amine
-
79-44-7
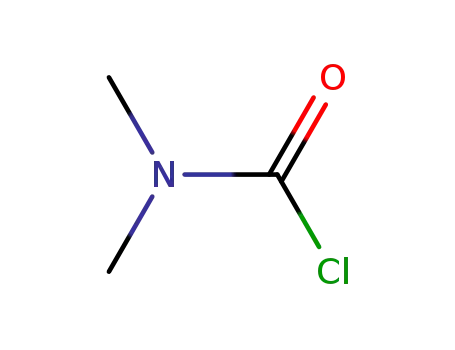
N,N-Dimethylcarbamoyl chloride
-
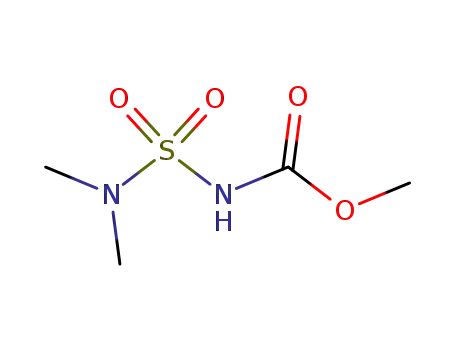
89168-09-2
Carbamidsaeure-methylester-N-sulfonsaeure-dimethylamid
13360-57-1 Downstream products
-
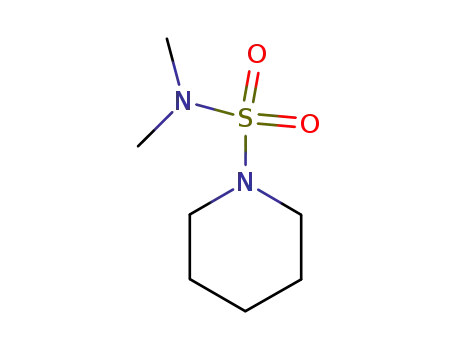
5417-33-4
piperidine-1-sulfonic acid dimethylamide
-
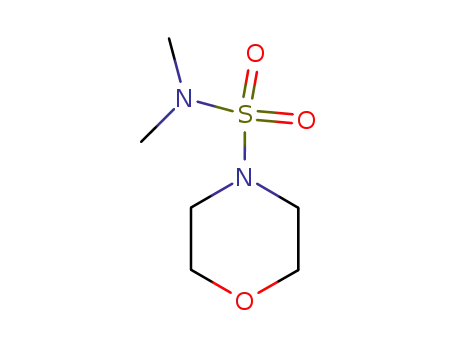
5433-57-8
N-Dimethylaminosulfonyl-morpholin
-
98961-97-8
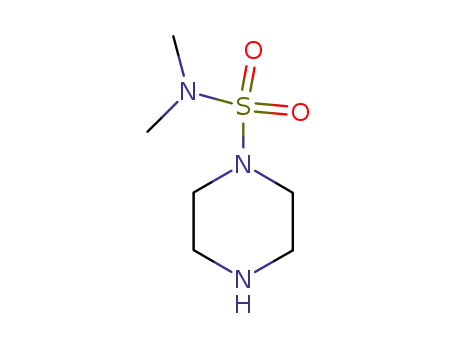
piperazine-1-sulfonic acid dimethylamide
-
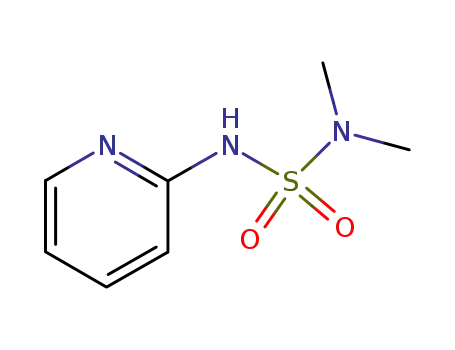
54767-77-0
N,N-dimethyl-N'-[2]pyridyl-sulfamide