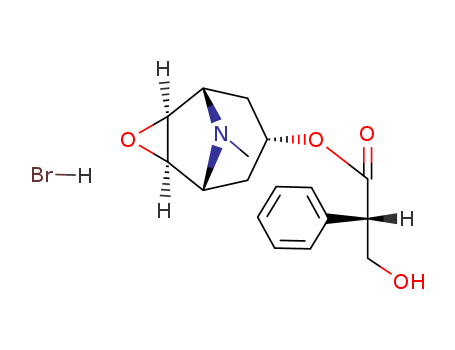

Product Details
|
Description |
Scopolamine is a tropane alkaloid that can be found in many plants of the Solanaceae (nightshade) family. It is a muscarinic receptor antagonist that can be used to induce memory impairment in animals. Scopolamine prevents motion sickness, nausea, and vomiting in animals. |
|
Chemical Properties |
Off-White Solid |
|
Uses |
An acetylcholine antagonist. Used in treatment of motion sickness; antiemetic; antispasmodic; mydriatic; preanesthetic medicant |
|
Definition |
ChEBI: A hydrobromide that is obtained by reaction of scopolamine with hydrogen bromide. |
|
Brand name |
Isopto Hyoscine (Alcon); Transderm-Scop (Ciba-Geigy). |
|
General Description |
Scopolamine hydrobromide(hyoscine hydrobromide) occurs as white orcolorless crystals or as a white, granular powder. It is odorlessand tends to effloresce in dry air. It is freely soluble inwater (1:1.5), soluble in alcohol (1:20), only slightly solublein chloroform, and insoluble in ether.Scopolamine is a competitive blocking agent of theparasympathetic nervous system as is atropine, but it differsmarkedly from atropine in its action on the higher nervecenters. Both drugs readily cross the blood-brain barrierand, even at therapeutic doses, cause confusion, particularlyin the elderly. |
|
Air & Water Reactions |
Sensitive to air, light and moisture. Water soluble. |
|
Reactivity Profile |
Scopolamine hydrobromide is incompatible with acids, bases and oxidizing agents. . |
|
Fire Hazard |
Flash point data for Scopolamine hydrobromide are not available; however, Scopolamine hydrobromide is probably combustible. |
|
Biological Activity |
Non-selective muscarinic antagonist. Widely used clinically to treat motion sickness. |
|
Clinical Use |
A sufficiently large dose of scopolamine will cause an individualto sink into a restful, dreamless sleep for about8 hours, followed by a period of approximately the samelength in which the patient is in a semiconscious state.During this time, the patient does not remember events thattake place. When scopolamine is administered with morphine,this temporary amnesia is termed twilight sleep. |
|
Drug interactions |
Potentially hazardous interactions with other drugs None known |
|
Metabolism |
Hyoscine hydrobromide is almost entirely metabolised, probably in the liver; only a small proportion of an oral dose is excreted unchanged in the urine. In one study in man, 3.4% of a single dose, administered by subcutaneous injection was excreted unchanged in urine within 72 hours. |
|
Purification Methods |
The hydrobromide is recrystallised from Me2CO, H2O or EtOH/Et2O and dried. It is soluble in H2O (60%) and EtOH (5%) but insoluble in Et2O and slightly in CHCl3. The hydrochloride has m 300o (from Me2CO). The free base is a viscous liquid which forms a crystalline hydrate with m 59o and [] D 20 -28o (c 2.7, H2O). It hydrolyses in dilute acid or base. [Meinwald J Chem Soc 712 1953, Fodor Tetrahedron 1 86 1957, Beilstein 6 III 4185.] |
InChI:InChI=1/C17H21NO4.BrH/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10;/h2-6,11-16,19H,7-9H2,1H3;1H/t11-,12-,13+,14?,15-,16?;/m1./s1
A process for preparing tropenol (I) or ...

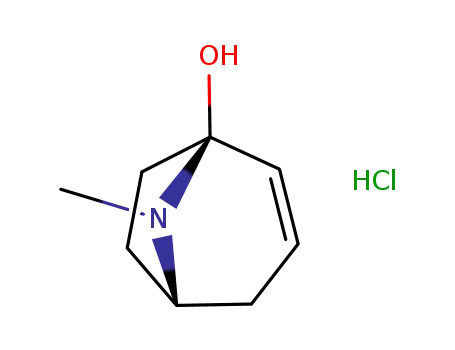
tropenol hydrochloride

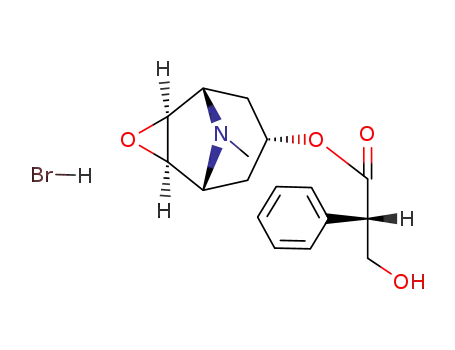
hyoscine hydrobromide
| Conditions | Yield |
|---|---|
|
|
81% |

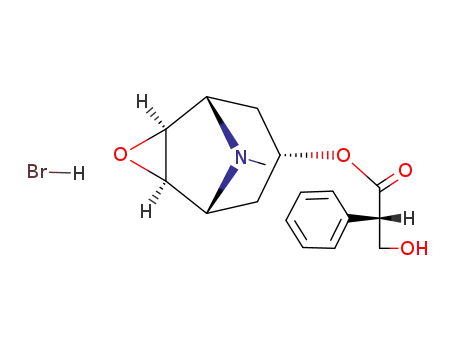
(+/-)-scopolamine; hydrobromide
| Conditions | Yield |
|---|---|
|
With water;
|
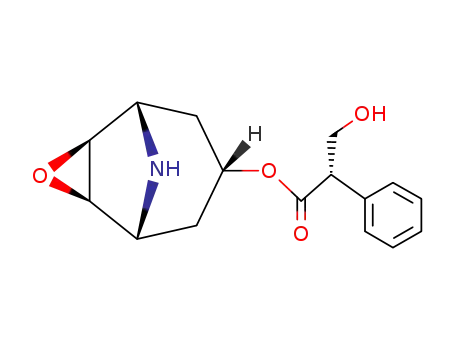
Norscopolamine
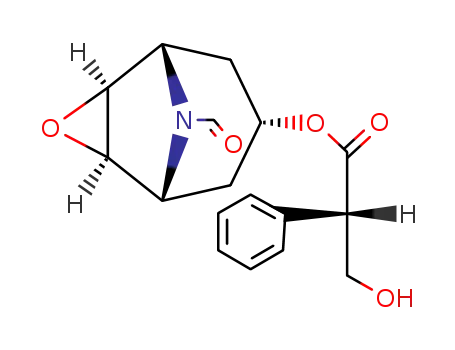
(-)-Formylnorscopollamin
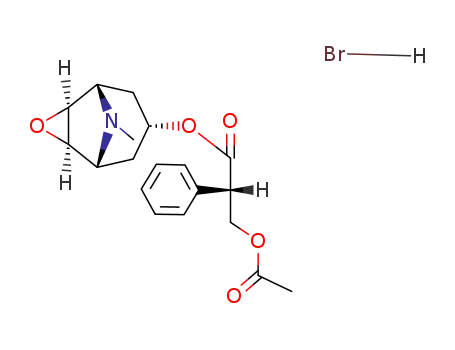
(-)-O-Acetylscopolamin-hydrobromid
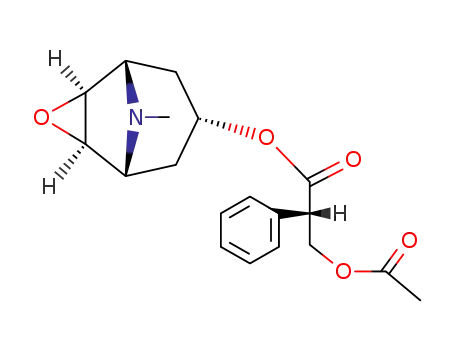
9-methyl-3-oxa-9-azatricyclo[3.3.1.0(2,4)]non-7-yl-3'-(acetyloxy)-2'-phenyl propanoate
CAS:51-74-1
CAS:886-86-2
CAS:505-66-8
CAS:405-05-0