Your Location:Home >Products >Fine chemicals >505-66-8

Product Details
|
Important pharmaceutical intermediates |
Homopiperazine is a nitrogen-containing seven-membered ring hetero-atomic compound and is an important pharmaceutical intermediate. The majority of its derivatives has strong biological activity and medicinal value and can be used for synthesizing homopiperazine hydrochloride, cyclizine, carbamazepine, chlorcyclizine and other drugs. Western medicine often takes homopiperazine as raw material for the modified quinoline and isoquinoline derivatives, quinolone derivative, thiazolidine carboxylic acid amide derivatives and other drugs, and for the synthesis of pyridazine, nitroxyl-containing benzylamine derivatives, water-soluble azole and other drugs which has good efficacy for the treatment of cardiovascular disease, interstitial plasma cell pneumonia especially for AIDS patients with Pneumocystis pneumonia, asthma, central nervous system disorders including depression and anxiety disorders. For example, 1-(5-isoquinoline-sulfonyl) homopiperazine hydrochloride is effective in the treatment of symptoms such as chronic angina disease, vitiligo, depression, anxiety, cerebral vasospasm and cerebral vasospasm caused cerebral ischemia and other symptoms. Homopiperazine and its high-piperazine derivatives have become important pharmaceutical intermediates and have exhibited great potential of being sedative, anti-psychotic, anti-inflammatory and anti-neurotic and is an important product of a connecting link between the proceeding and the following in the chemical industry and pharmaceutical industry and have been widely applied to the pharmaceuticals, pesticides, surfactants, energetic materials and other fields. Currently the domestic heterocyclic compounds are mainly used for producing the quinolone antibacterial drugs. This drug, through inhibiting the bacterial DNA gyrase, blocks the DNA replication and produces the antimicrobial effects. According to the report of ARMIGER H et al, after using homopiperazine as substitute of piperazine for the synthesis of drugs like cyclizine, homo-chlorcyclizine and other drugs, animal experiments had demonstrated that the activity of antihistamine drugs had been significantly enhanced. Its main products include quinoline and isoquinoline derivatives, quinolone derivative, thiazolidine carboxylic acid amide derivatives and other drugs, as well as the synthesis of disubstituted pyridazine, the nitroxyl-containing benzyl amine derivatives and water-soluble azole drug. The derivative of homopiperazine molecule with the hydrogen atom in the amino group being substituted by long-chain alkyl or alkoxy can be used as wetting agents, emulsifying agents, detergents, coloring agents; homopiperazine and its derivatives, because of containing staining keratin filament, can be used as oxidation coloring agents and have vibrant colors and high solidness properties. It plays an important role in the dyeing, especially in the dyeing of human hair. Polymers containing homopiperazine monomer have many unique properties such as being able to improve the melting point and improve their solubility. Resin and synthetic fiber containing such a polymer have been applied to a variety of special fields. The above information is edited by the lookchem of Dai Xiongfeng. |
|
Preparation |
Homopiperazine can be synthesized using amino compounds such as N-(2-cyanoethyl)-ethylenediamine, N-(β-hydroxy)-1,3-propanediamine, ethylenediamine as raw material so that homopiperazine has various kinds of synthetic routes according to different raw materials. With the development of China's petrochemical industry, for the synthesis of the starting raw material of homopiperazine, alcohols and ethylene diamine, it not only has low price but also can get high-quality product as well as extremely abundant source. Currently, though there are homopiperazine domestic manufacturers, but the technology is lagged behind, the quality is not high and the production output is limited, all the above points are in urgent need for being transformed and improved. From this perspective, it is imperative for accelerating the process of development of fine chemical products of homopiperazine with a wide range of application. Therefore, the market prospect of the homopiperazine is very broad. Using ethylenediamine as the raw material: the method uses the easily available ethylenediamine as the initial raw material, further goes through sulfonylation, cyclization, de-sulfonylation, 3-step reaction for synthesizing homopiperazine with the total yield being 78%. Within this synthetic method, during the first step of ethylenediamine sulfonylation, the addition of a phase transfer catalyst can significantly improve the product yield with the yield being 86%. During the second step of cyclization reaction, selection of NaH/ DMF reaction system can enable the completion of the cyclization reaction under milder reaction conditions. Finally, under HBr/HAc/PhOH reaction conditions, remove the sulfonyl group with a yield being 91% to give the final product homopiperazine. The results indicate that it is an excellent homopiperazine synthesis route of various kinds of advantages including easily available raw materials, simple operation and high yield with certain prospects for industrial application. |
|
Uses |
It can be used as organic and pharmaceutical synthesis intermediates, for example, being applied to the synthesis of fasudil hydrochloride. Homopiperazine acts as a corrosion inhibitor for iron. It has also been used in the preparation of potent H3 receptor antagonists for use as treatments for neurodegenerative conditions such as Alzheimer disease. |
|
Chemical Properties |
white to light yellow crystalline mass |
InChI:InChI=1/C5H12N2/c1-2-6-4-5-7-3-1/h6-7H,1-5H2/p+2
The titled compound 1,4-diazacycloheptan...
An efficient, robust, and cost-effective...
The invention discloses a cardiovascular...
The invention discloses a method for pre...
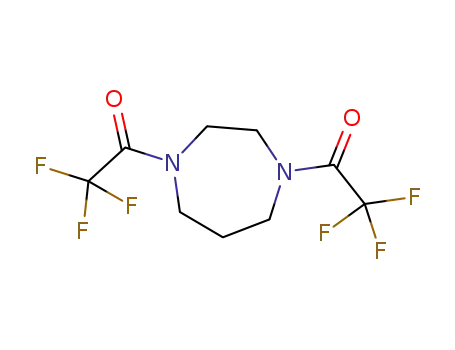
1,4-bistrifluoroacetyl homopiperazine

1,4-Diazacycloheptane
| Conditions | Yield |
|---|---|
|
1,4-bistrifluoroacetyl homopiperazine; With hydrogenchloride; In ethanol; at 40 ℃; for 5h; Autoclave; Large scale;
With sodium hydroxide; In water; toluene; at 20 ℃; for 1h; Autoclave; Large scale;
|
83.05% |
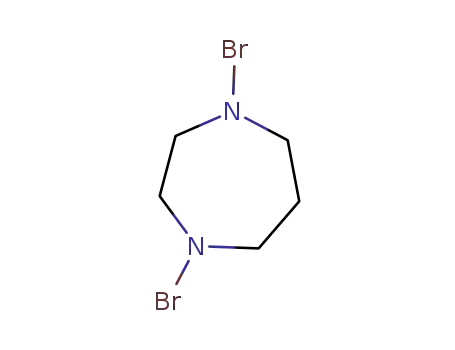
C5H10Br2N2


1,4-Diazacycloheptane
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In toluene; Autoclave; Reflux; Large scale;
|
85% |
2-[(3-aminopropyl)amino]ethanol
N,N'-Di(phenylsulphonyl)hexahydro-1,4-diazepin
ethylene glycol
Trimethylenediamine
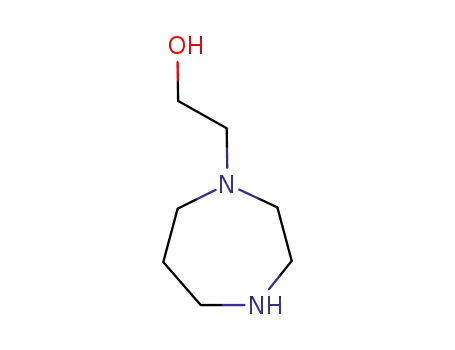
2-(1,4-diazepan-1-yl)ethanol
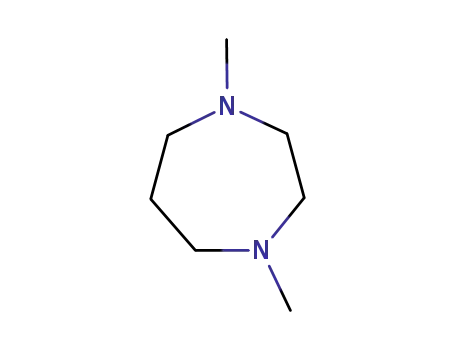
1,4-dimethylhexahydro-1,4-diazepine

N,N'-Di(phenylsulphonyl)hexahydro-1,4-diazepin
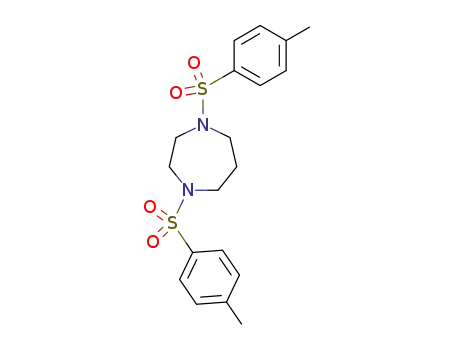
N,N'-di-p-toluenesulfonylhomopiperazine
CAS:2941-78-8
CAS:1143516-05-5
CAS:1235479-59-0
CAS:114-49-8