Your Location:Home >Products >Intermediates >241479-73-2
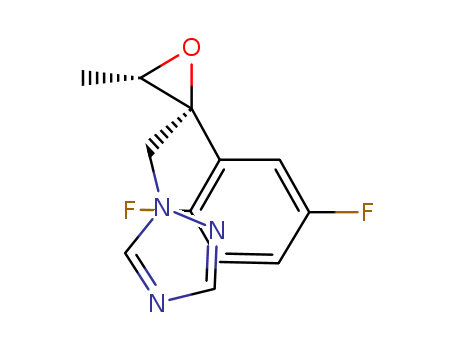

Product Details
An enantioselective epoxidation of α-sub...
A compound represented by formula (II): ...
The invention discloses a synthetic meth...
The present invention relates to a proce...
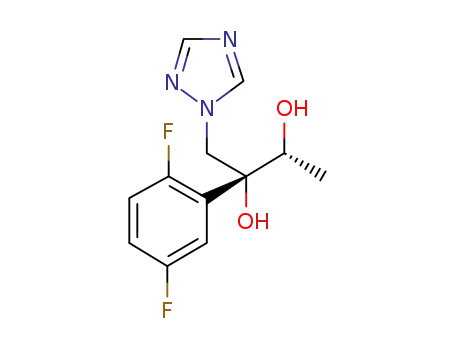
(2R,3R)-2-(2,5-difluoro-phenyl)-1-(1H-1,2,4-triazole-1-yl)butan-2,3-diol

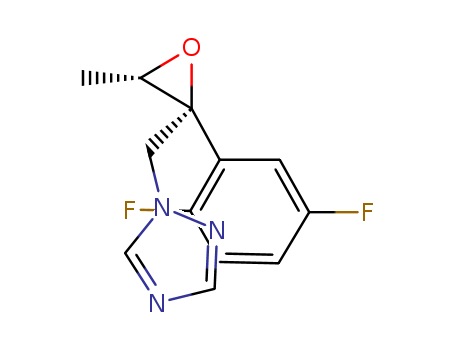
![(2R,3S)-2-(2,5-difluorophenyl)-3-methyl-2-[(1H-1,2,4-triazol-1-yl)-methyl]-oxirane](/upload/2024/4/92aee102-c49a-478d-9f81-aa540ed2dcd7.png)
(2R,3S)-2-(2,5-difluorophenyl)-3-methyl-2-[(1H-1,2,4-triazol-1-yl)-methyl]-oxirane
| Conditions | Yield |
|---|---|
|
(2R,3R)-2-(2,5-difluoro-phenyl)-1-(1H-1,2,4-triazole-1-yl)butan-2,3-diol; With methanesulfonyl chloride; triethylamine; In tetrahydrofuran; at 0 ℃; for 0.333333h;
With tetrabutylammomium bromide; sodium hydroxide; In tetrahydrofuran; water; at 20 ℃; for 12h; enantioselective reaction;
|
95% |
|
With sodium methylate; triethylamine; In methanol; CH2Cl2-EtOAc; ethyl acetate; methanesulfonyl chloride;
|
88% |
|
With tert-butyl methyl ether; methanesulfonyl chloride; triethylamine; In tetrahydrofuran; at -10 - 10 ℃; for 1h; Large scale;
|
85% |
|
With methanesulfonyl chloride; triethylamine; In dichloromethane; at 10 - 25 ℃;
|
33.5 g |
|
With methanesulfonyl chloride; triethylamine; at 0 - 3 ℃; for 3h;
|
5.94 g |

(2R,3R)-2-(2,5-difluoro-phenyl)-1-(1H-1,2,4-triazole-1-yl)butan-2,3-diol

NaOMe methanol

![(2R,3S)-2-(2,5-difluorophenyl)-3-methyl-2-[(1H-1,2,4-triazol-1-yl)-methyl]-oxirane](/upload/2024/4/92aee102-c49a-478d-9f81-aa540ed2dcd7.png)
(2R,3S)-2-(2,5-difluorophenyl)-3-methyl-2-[(1H-1,2,4-triazol-1-yl)-methyl]-oxirane
| Conditions | Yield |
|---|---|
|
With triethylamine; In methanol; dichloromethane;
|
93% |
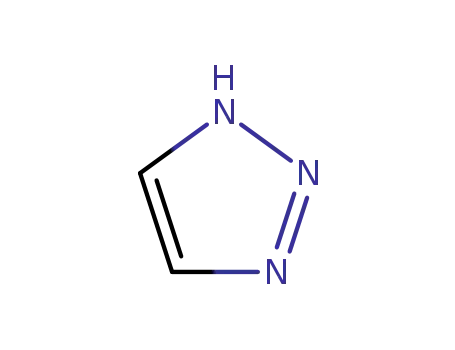
1,2,3-triazole
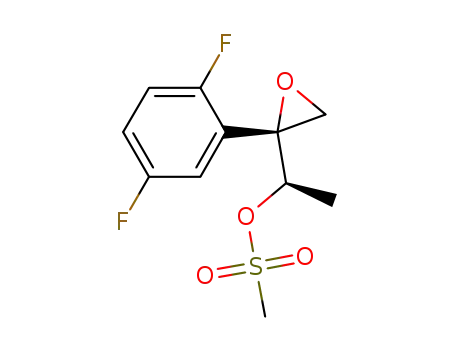
(R)-1-[(R)-2-(2,5-difluorophenyl)-2-oxiranyl]ethyl methanesulfonate

(2R,3R)-2-(2,5-difluoro-phenyl)-1-(1H-1,2,4-triazole-1-yl)butan-2,3-diol
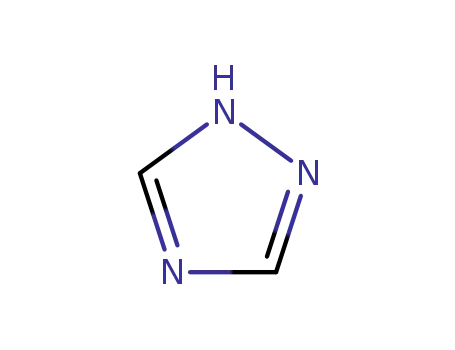
1,2,4-Triazole
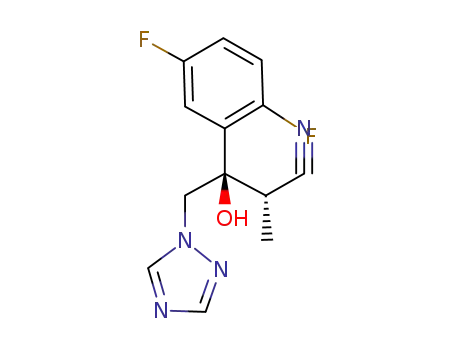
(2S,3R)-3-(2,5-difluorophenyl)-3-hydroxy-2-methyl-4-(1H-1,2,4-triazol-1-yl)butyronitrile
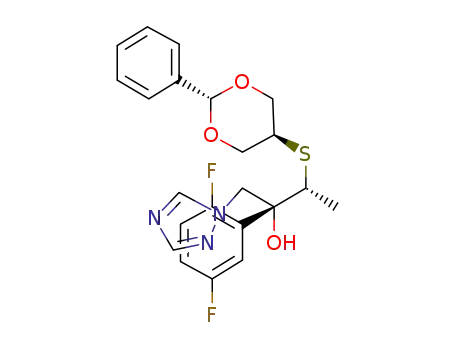
(2R,3R)-2-(2,5-difluorophenyl)-3-[(trans-2-phenyl-1,3-dioxan-5-yl)thio]-1-(1H-1,2,4-triazol-1-yl)-2-butanol
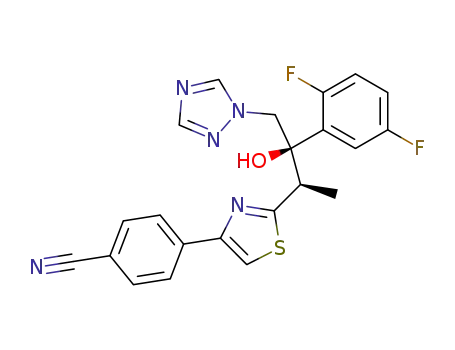
Isavuconazole
CAS:50-91-9
CAS:39065-95-7
CAS:21962-45-8
CAS:1235479-59-0