Your Location:Home >Products >Fine chemicals >50-91-9
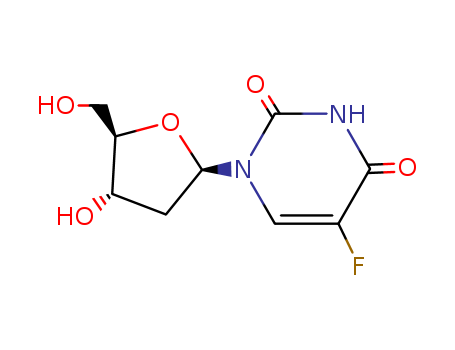

Product Details
5'-Dipeptidyl derivatives of 5-fluorodeo...
-
Floxuridine oligomers are anticancer oli...
An efficient and green bioprocess is her...
Nucleoside analogs represent a class of ...
The present invention relates to a proce...
Unnatural nucleosides are attracting int...
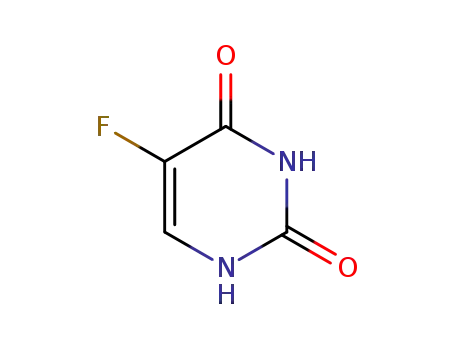
5-fluorouracil

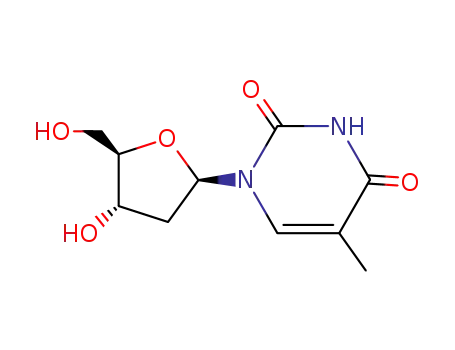
thymidine

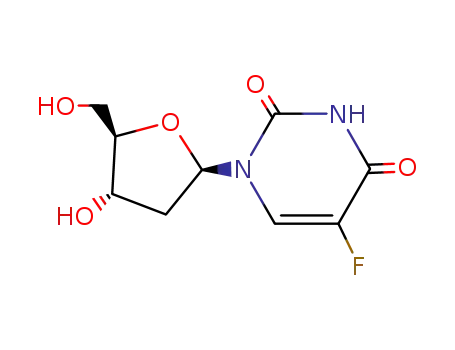
5-Fluoro-2'-deoxyuridine
| Conditions | Yield |
|---|---|
|
With Lactobacillus animalis ATCC 35046 2’-N-deoxyribosyltransferase immobilized in DEAE-Sepharose; In aq. buffer; at 30 ℃; pH=7; Concentration; Reagent/catalyst; Green chemistry; Enzymatic reaction;
|
35% |
|
With thymidine phosphorylase; In aq. phosphate buffer; at 37 ℃; for 0.2h; pH=6.8; Enzymatic reaction;
|
|
|
With ammonium dihydrogen phosphate; ammonia; In water; at 45 - 55 ℃; for 45h; pH=6.6; Large scale; Enzymatic reaction;
|
|
|
With recobinant purine nucleoside phosphorylase from Escherichia coli; In aq. phosphate buffer; pH=6.8; Heating; Enzymatic reaction;
|
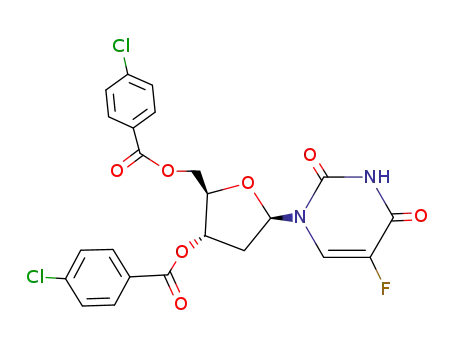
3',5'-di-O-(p-chlorobenzoyl)-5-fluoro-2'-deoxy-β-uridine


5-Fluoro-2'-deoxyuridine
| Conditions | Yield |
|---|---|
|
With methanol; ammonia; at 10 - 25 ℃;
|
86.2% |
|
With ammonia; In methanol; at 30 ℃; for 16h;
|
|
|
With methanol; barium methoxide;
|
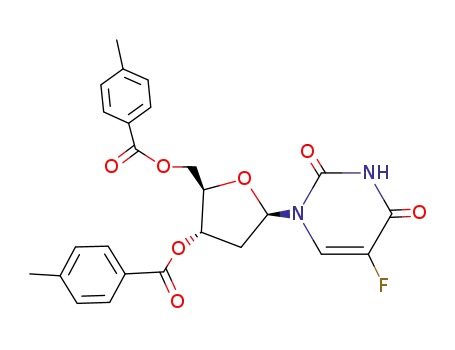
5-fluoro-O3',O5'-bis-(4-methyl-benzoyl)-2'-deoxy-uridine

3',5'-di-O-(p-chlorobenzoyl)-5-fluoro-2'-deoxy-β-uridine
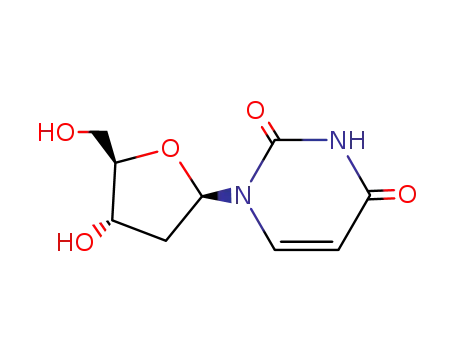
2'-deoxyuridine
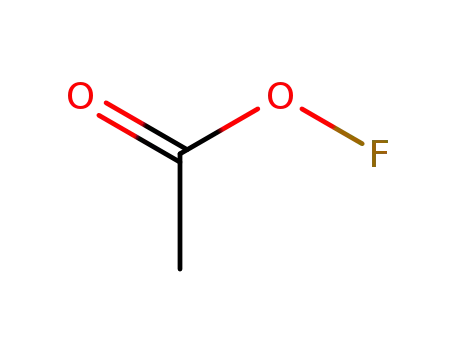
acetyl hypofluorite
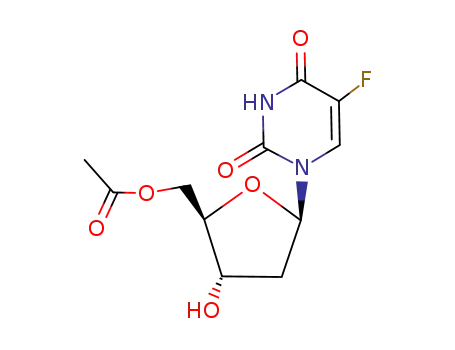
5'-O-acetyl-floxuridine
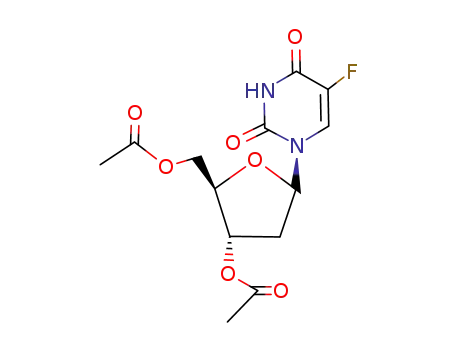
Acetic acid (2R,3S)-2-acetoxymethyl-5-((S)-5-fluoro-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-tetrahydro-furan-3-yl ester
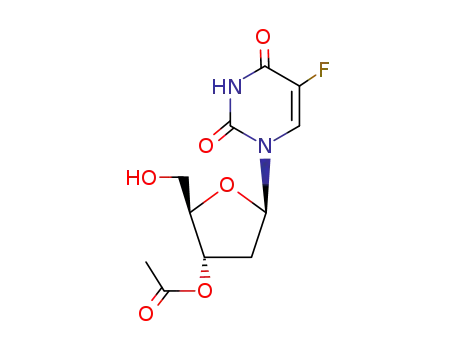
3′-O-acetyl-5-fluoro-2′-deoxyuridine
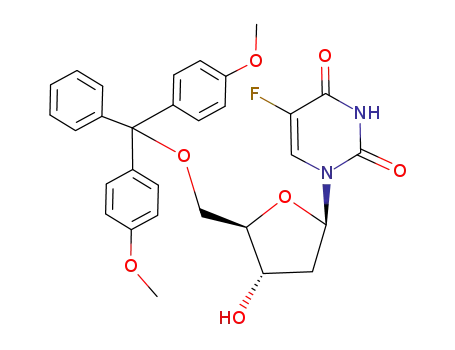
1-((2R,4S,5R)-5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-4-hydroxytetrahydrofuran-2-yl)-5-fluoropyrimidine-2,4(1H,3H)-dione
CAS:39065-95-7
CAS:18871-66-4
CAS:2973-59-3