Your Location:Home >Products >Fine chemicals >18871-66-4

Product Details
|
Chemical Properties |
clear yellow to brown liquid |
|
Uses |
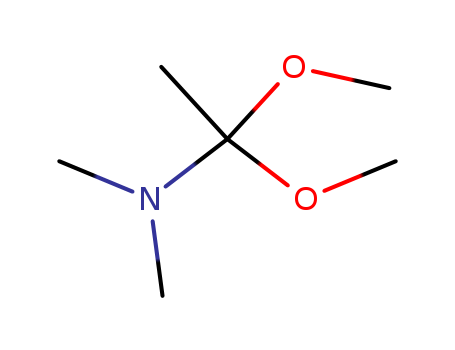
N,N-Dimethylacetamide dimethyl acetal has been used as:one carbon inserting synthon in preparation of pyrimido[1,2-a][1,3,5]triazin-6-onesreagent for the synthesis of amides, diacylamines and heterocycles |
|
General Description |
Claisen type reaction of N,N-dimethylacetamide dimethyl acetal using optically active trans-3-penten-2-ol as substrate has been investigated. |
InChI:InChI=1/C6H15NO2/c1-6(8-4,9-5)7(2)3/h1-5H3/p+1
A series of novel amidine derivatives of...
N,N-Dimethylacetamide (DMAc, 1)/lithium ...
An efficient method for the synthesis of...
-
Indole derivatives and mono- or diazaind...
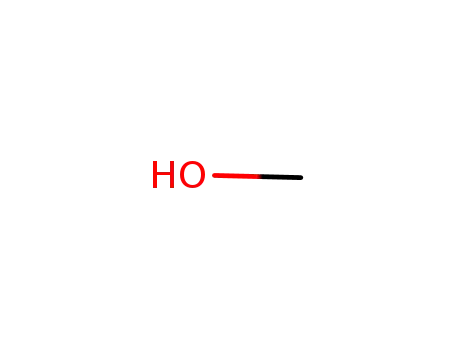
methanol

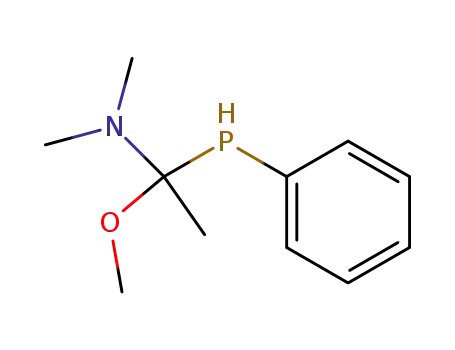
(1-Methoxy-1-phenylphosphanyl-ethyl)-dimethyl-amine

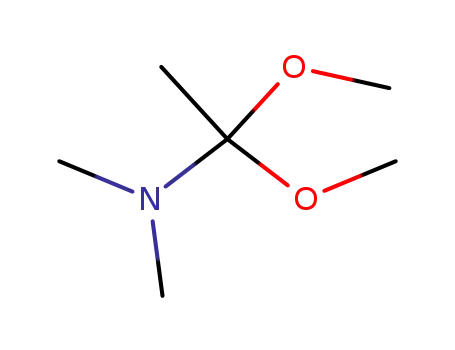
N,N-dimethylacetamide dimethyl acetal

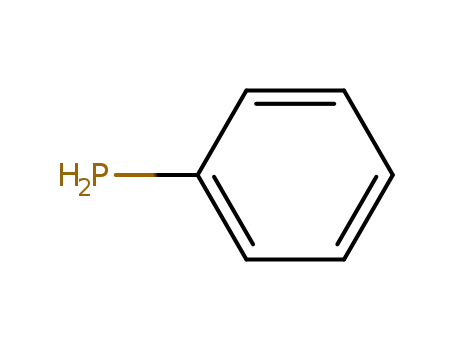
phenylphosphane
| Conditions | Yield |
|---|---|
|
In acetonitrile;
|

methanol

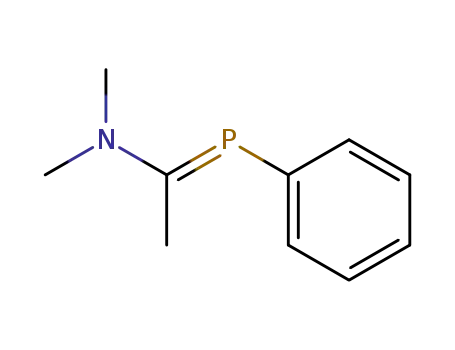
N,N-dimethylamino-P-phenyl-C-methylmethylenephosphine


N,N-dimethylacetamide dimethyl acetal


(1-Methoxy-1-phenylphosphanyl-ethyl)-dimethyl-amine

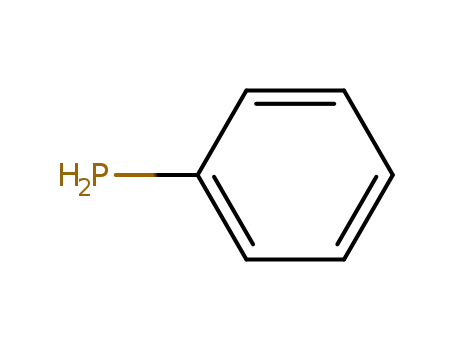
phenylphosphane
| Conditions | Yield |
|---|---|
|
In acetonitrile; Heating;
|

methanol

N,N-dimethylamino-P-phenyl-C-methylmethylenephosphine

(1-Methoxy-1-phenylphosphanyl-ethyl)-dimethyl-amine
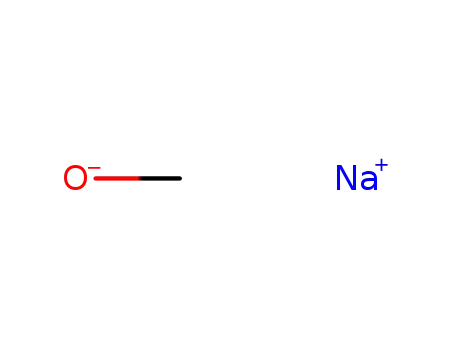
sodium methylate
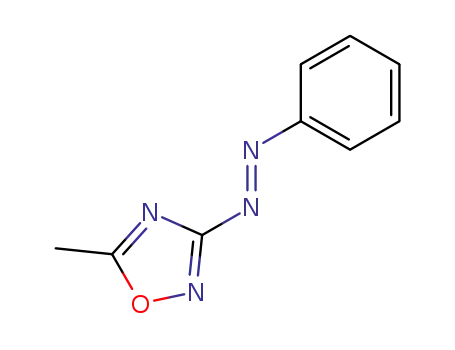
5-methyl-3-trans-phenylazo-[1,2,4]oxadiazole
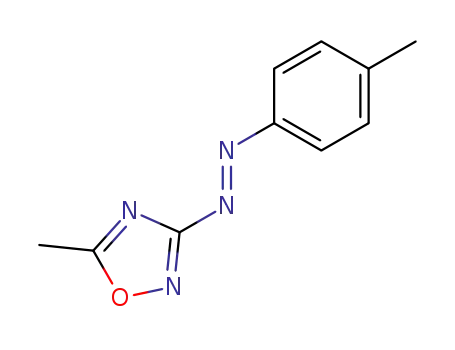
5-methyl-3-trans-p-tolylazo-[1,2,4]oxadiazole
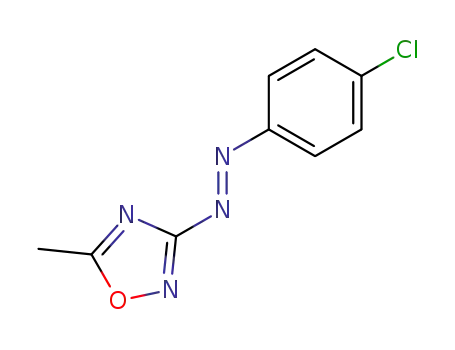
3-(4-chloro-trans-phenylazo)-5-methyl-[1,2,4]oxadiazole
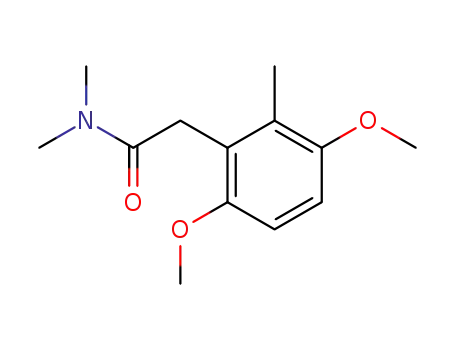
2-(3,6-Dimethoxy-2-methyl-phenyl)-N,N-dimethyl-acetamide
CAS:2941-78-8
CAS:1143516-05-5
CAS:886-86-2
CAS:50-91-9