Your Location:Home >Products >Intermediates >5118-13-8
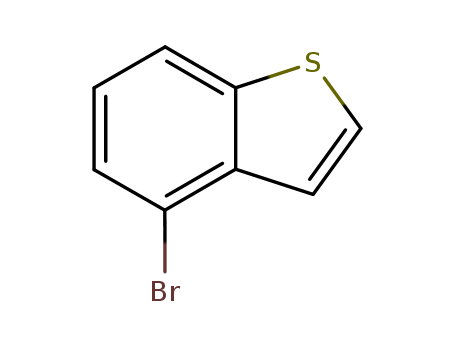

Product Details
|
Synthesis |
The traditional synthesis method is to take 2-bromo-6-fluorobenzaldehyde as a raw material, cyclize the raw material with mercaptoacetic acid in a DMF solvent to obtain 4-chlorobenzo [b] thiophene-2-carboxylic acid, and then decarboxylate the product at a high temperature in a quinoline/copper powder system to obtain the 4-chlorobenzo [b] thiophene. |
|
Uses |
4-bromobenzo [b] thiophene is an important intermediate in the drug ipiprazole for the treatment of schizophrenia. |
InChI:InChI=1/C8H5BrS/c9-7-2-1-3-8-6(7)4-5-10-8/h1-5H
The invention belongs to the field of ch...
The base-catalyzed isomerization of simp...
The invention discloses a novel synthesi...
The invention discloses a synthesis meth...
![4-bromo-benzo[b]thiophene-2-carboxylic acid](/upload/2024/4/4e3bf838-f364-4cca-8213-f452dac68e98.png)
4-bromo-benzo[b]thiophene-2-carboxylic acid

![4-bromobenzo[b]thiophene](/upload/2024/4/5362b45a-b7b1-4b77-a097-e50e6ae81000.png)
4-bromobenzo[b]thiophene
| Conditions | Yield |
|---|---|
|
With acetic acid; silver carbonate; In dimethyl sulfoxide; at 120 ℃; Further stages;
|
95% |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In N,N-dimethyl acetamide; at 200 ℃; for 1h; microwave irradiation;
|
91% |
|
With triethylenediamine; at 120 - 180 ℃; for 6h;
|
91.7% |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In sulfolane; at 300 ℃; for 0.666667h; Inert atmosphere;
|
81% |
|
4-bromo-benzo[b]thiophene-2-carboxylic acid; With quinoline; copper; at 195 ℃; for 5h;
With hydrogenchloride; water; at 0 ℃;
|
37% |
|
copper; In quinoline; at 190 ℃; for 1h;
|
|
|
With copper;
|
![Benzo[b]thiophene](/upload/2024/4/4e44b4ed-a6be-4514-8284-731b6ab731af.png)
Benzo[b]thiophene

![4-bromobenzo[b]thiophene](/upload/2024/4/5362b45a-b7b1-4b77-a097-e50e6ae81000.png)
4-bromobenzo[b]thiophene
| Conditions | Yield |
|---|---|
|
Benzo[b]thiophene; With dihydrogen peroxide; acetic acid; at 78 ℃; for 0.5h; Inert atmosphere;
With tetrabutylammomium bromide; sodium bromide; In water; at 120 ℃; for 20h; under 7220.48 Torr;
|
98.8% |
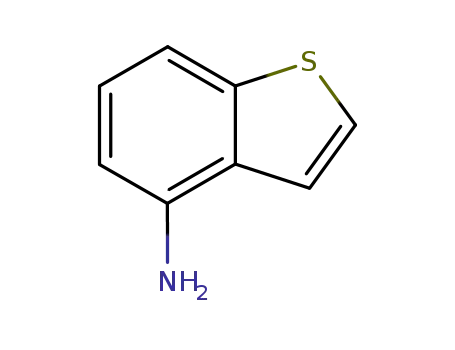
benzo[B]thiophen-4-ylamine
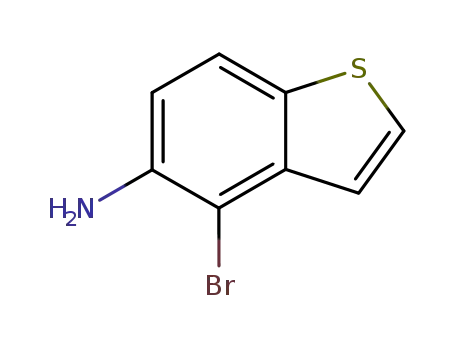
4-bromo-5-aminobenzothiophene
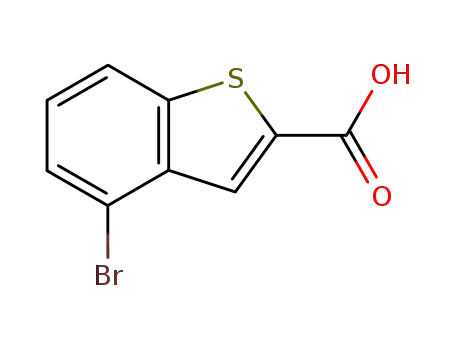
4-bromo-benzo[b]thiophene-2-carboxylic acid
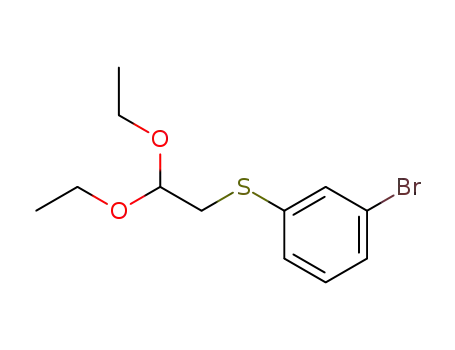
1-bromo-3-(2,2-diethoxy-ethylsulfanyl)benzene
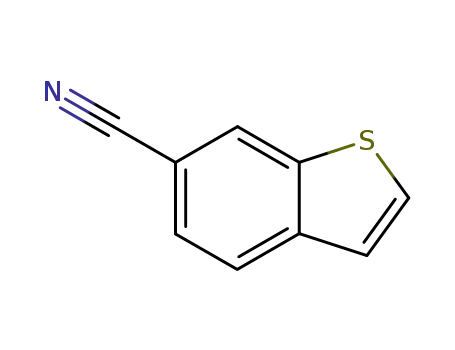
benzo[b]thiophene-6-carbonitrile
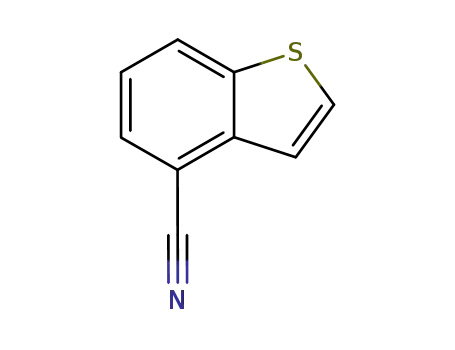
benzo[b]thiophene-4-carbonitrile
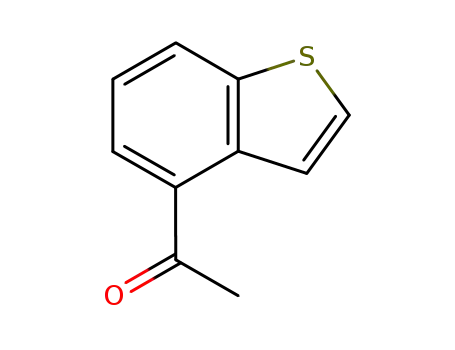
4-acetylbenzothiophen
4-[(E)-2-phenylvinyl]benzo[b]thiophene
CAS:131707-23-8
CAS:6482-24-2
CAS:552-16-9