Your Location:Home >Products >Intermediates >552-16-9
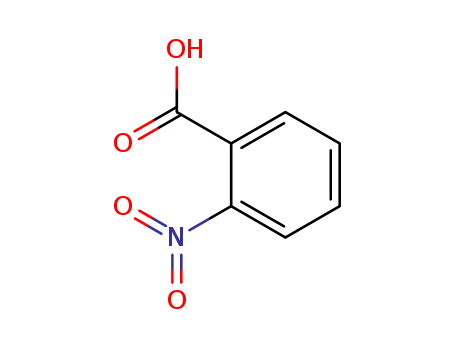

Product Details
|
Chemical Properties |
off-white powder |
|
Uses |
NH2-protecting reagent. |
|
Definition |
ChEBI: A nitrobenzoic acid carrying the nitro substituent at position 2. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 56, p. 5924, 1991 DOI: 10.1021/jo00020a040Tetrahedron Letters, 35, p. 219, 1994 DOI: 10.1016/S0040-4039(00)76515-4 |
|
General Description |
Yellowish white crystals with an intensely sweet taste. |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
2-Nitrobenzoic acid is incompatible with strong oxidizing agents. Also incompatible with strong bases. May react with cyanides . |
|
Health Hazard |
ACUTE/CHRONIC HAZARDS: When 2-Nitrobenzoic acid is heated to decomposition it emits very toxic fumes. |
|
Fire Hazard |
Flash point data concerning 2-Nitrobenzoic acid are not available, however, 2-Nitrobenzoic acid is probably combustible. |
|
Purification Methods |
Crystallise the acid from *benzene (twice), n-butyl ether (twice), then water (twice). Dry and store it in a vacuum desiccator. [Le Noble & Wheland J Am Chem Soc 80 5397 1958.] It has also been crystallised from EtOH/H2O. The amide has m 176.5o (from H2O). [Beilstein 9 III 1466, 9 IV 1046.] |
InChI:InChI=1/C7H5NO4/c9-7(10)5-3-1-2-4-6(5)8(11)12/h1-4H,(H,9,10)/p-1
CO2 fixation into electron-deficient aro...
A variety of deactivated arenes were nit...
The photocatalytic carboxylation of aryl...
The invention relates to the technical f...
o-nitrobenzonitrile

ortho-nitrobenzoic acid

2-nitrobenzamide
| Conditions | Yield |
|---|---|
|
With phosphate buffer; at 30 ℃; for 4h; rhodococcus rhodocrous AJ270, pH 7.0;
|
10% 90% |
|
With potassium phosphate buffer; at 30 ℃; for 4h; Rhodococcus sp. AJ270 cells;
|
10% 89.5% |
1-methyl-2-nitrobenzene


ortho-nitrobenzoic acid

2-nitrobenzyl chloride
| Conditions | Yield |
|---|---|
|
With sodium hypochlorite; bis(2,2'-bipyridine)dichloronickel(II); In acetonitrile; for 1h; Yield given; Yields of byproduct given; Ambient temperature;
|
|
|
With hydrogenchloride; sodium hypochlorite; tetra(n-butyl)ammonium hydrogensulfate; In dichloromethane; water; at 20 ℃; for 49h; pH=9; Further Variations:; Reagents; various reagent ratios, reaction times; Product distribution;
|
pyridine
bis-(2-nitro-benzoyl)-peroxide
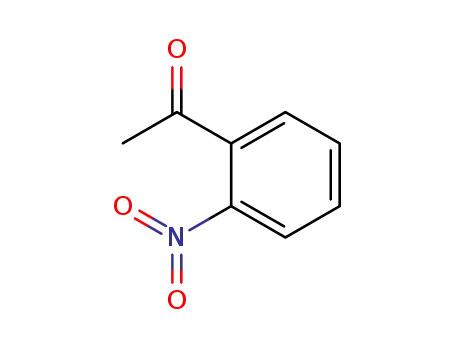
2-acetylnitrobenzene

1-methyl-2-nitrobenzene

2-nitrobenzamide
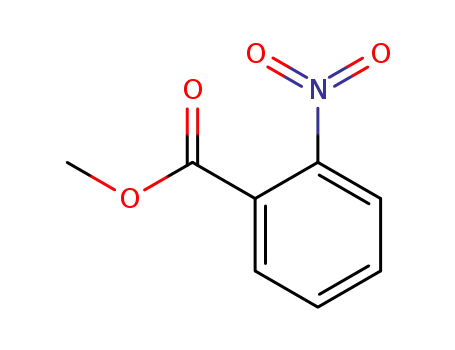
methyl 2-nitrobenzoate
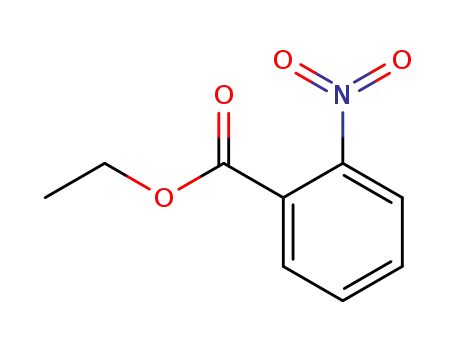
ethyl 2-nitrobenzoate
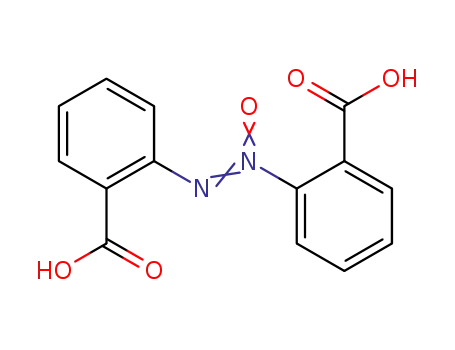
2,2'-azoxy-di-benzoic acid
CAS:2941-78-8
CAS:1143516-05-5
CAS:5118-13-8
CAS:77086-38-5