Your Location:Home >Products >Fine chemicals >77086-38-5
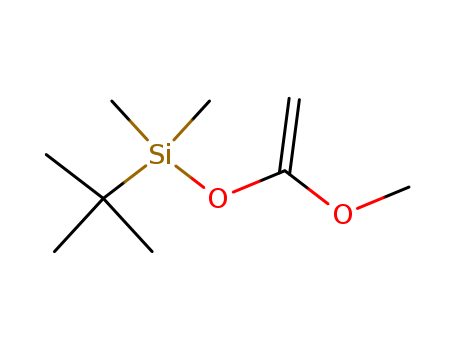

Product Details
|
General Description |
1-(tert-butyldimethylsilyloxy)-1-propene is a chemical compound that contains a tert-butyldimethylsilyloxy group and a propene group. It is commonly used as a building block in organic synthesis and serves as a protecting group for alcohols in chemical reactions. The tert-butyldimethylsilyloxy group provides steric hindrance and stability to the alcohol, allowing for selective manipulation of other functional groups within the molecule. 1-(TERT-BUTYLDIMETHYLSILYLOXY)-1- is often employed in the synthesis of complex organic molecules and pharmaceutical intermediates due to its versatile reactivity and ease of removal under mild conditions. Its use in synthetic chemistry has led to advancements in the development of new drugs and materials. |
InChI:InChI=1/C9H20O2Si/c1-8(10-5)11-12(6,7)9(2,3)4/h1H2,2-7H3
Nucleophilic addition reactions of soft ...
Mukaiyama–Mannich reactions of ester eno...
The Mukaiyama aldol reaction is a widely...
Tetratrifylpropene (TTP) has been develo...
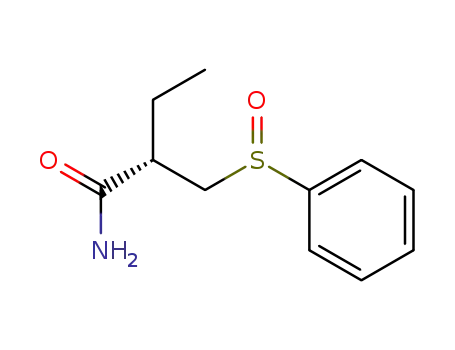
(2S)-2-(phenylsulfinylmethyl)butanamide

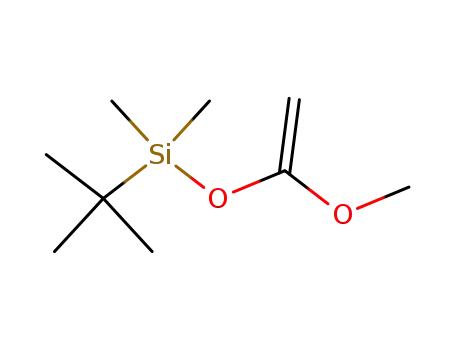
ketene t-butyldimethylsilyl methyl acetal

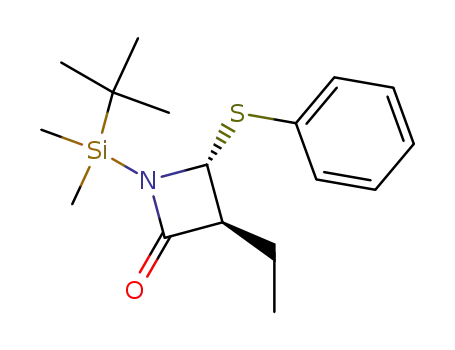
(3S,4R)-1-(tert-Butyl-dimethyl-silanyl)-3-ethyl-4-phenylsulfanyl-azetidin-2-one
| Conditions | Yield |
|---|---|
|
With zinc(II) iodide; In acetonitrile; at 70 ℃; for 8h; Yield given. Yields of byproduct given;
|
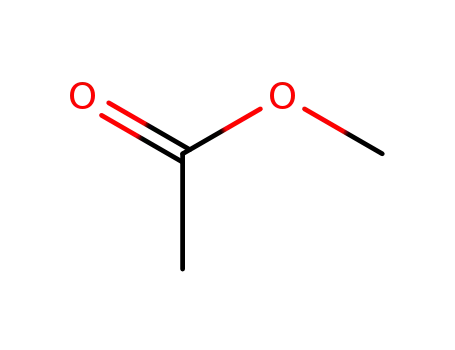
acetic acid methyl ester

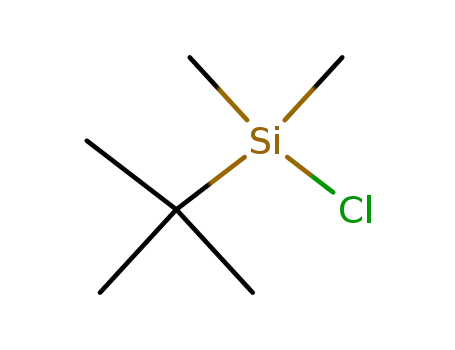
tert-butyldimethylsilyl chloride


ketene t-butyldimethylsilyl methyl acetal
| Conditions | Yield |
|---|---|
|
acetic acid methyl ester; With n-butyllithium; diisopropylamine; In tetrahydrofuran; hexane; at 0 ℃; for 0.333333h;
tert-butyldimethylsilyl chloride; With 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; In tetrahydrofuran; hexane; at -78 - 20 ℃; Further stages.;
|
86% |
|
acetic acid methyl ester; With N,N,N,N,N,N-hexamethylphosphoric triamide; n-butyllithium; diisopropylamine; In tetrahydrofuran; hexane; at -78 - 0 ℃; for 0.5h; Inert atmosphere;
tert-butyldimethylsilyl chloride; In tetrahydrofuran; hexane; at -78 - 0 ℃; for 2h; Inert atmosphere;
|
79% |
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; lithium diisopropyl amide; 1.) THF, -78 deg C, 75 min, 2.) n-pentane, 1 h;
|
72% |
|
acetic acid methyl ester; With n-butyllithium; diisopropylamine; In tetrahydrofuran; hexane; at -78 ℃; for 0.833333h;
With tris(pyrrolidino)phosphine oxide; In tetrahydrofuran; hexane; at -78 ℃; for 0.166667h;
tert-butyldimethylsilyl chloride; In tetrahydrofuran; hexane; at -78 ℃; for 1h;
|
65% |
|
acetic acid methyl ester; With n-butyllithium; diisopropylamine; In tetrahydrofuran; hexane; at -78 ℃; for 0.666667h;
tert-butyldimethylsilyl chloride; In tetrahydrofuran; 1-methyl-pyrrolidin-2-one; hexane; at -78 - 20 ℃; for 1.5h;
|
65% |
|
acetic acid methyl ester; With n-butyllithium; diisopropylamine; In tetrahydrofuran; at -78 ℃; for 0.666667h;
tert-butyldimethylsilyl chloride; In tetrahydrofuran; at -78 - 20 ℃;
|
58.3% |
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; lithium diisopropyl amide; Yield given. Multistep reaction; 1.) THF, hexane, -78 deg C, 2.5 min, 2.) THF, hexane, RT, 30 min;
|
|
|
acetic acid methyl ester; With lithium diisopropyl amide; In tetrahydrofuran; at -78 ℃; for 0.5h; Inert atmosphere;
tert-butyldimethylsilyl chloride; at -78 ℃; for 0.5h; Inert atmosphere;
|

(2S)-2-(phenylsulfinylmethyl)butanamide

acetic acid methyl ester
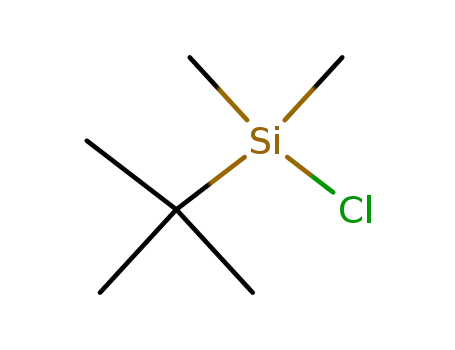
tert-butyldimethylsilyl chloride
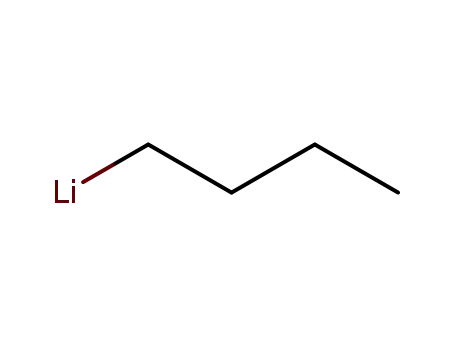
n-butyllithium
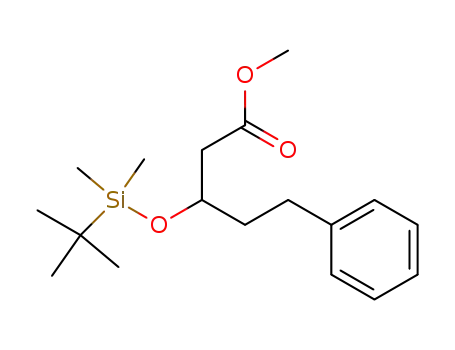
methyl 3-(t-butyldimethylsilyloxy)-5-phenylpentanoate
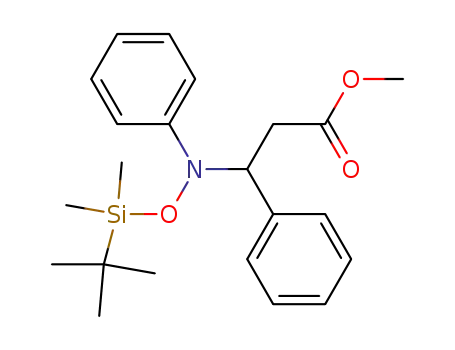
C22H31NO3Si
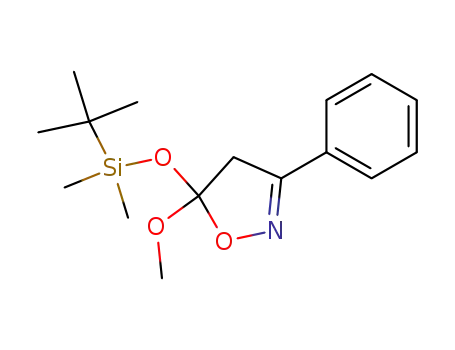
3-phenyl-5-(tert-butyldimethylsiloxy)-5-methoxyisoxazoline
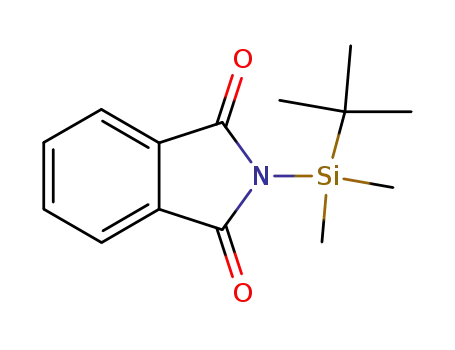
N-(tert-butyldimethylsilyl)phthalimide
CAS:14867-28-8
CAS:141-63-9
CAS:552-16-9
CAS:540-63-6