Your Location:Home >Products >Intermediates >1074-36-8
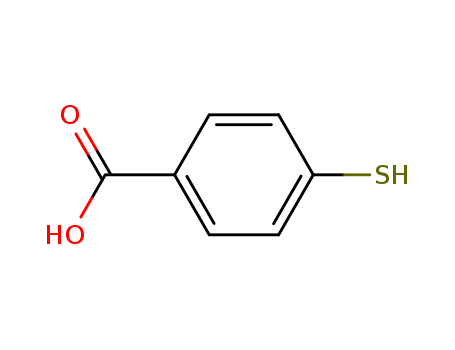

Product Details
|
Chemical Properties |
Cream Colour Crystalline Solid |
|
Uses |
Gold nanostructures functionalized with 4-Mercaptobenzoic acid (4-MBA) may exhibit improve catalytic activity. Several studies report 4-MBA as a model analyte for surface enhancement Raman scattering (SERS) substrates. |
|
General Description |
4-mercaptobenzoic acid (4-MBA) is a probe molecule with thiol and carboxylic groups that form a self-assembled monolayers (SAMs) which can be used for the development of surface enhanced raman spectroscopy (SERS) sensors. |
InChI:InChI=1/C7H6O2S/c8-7(9)5-1-3-6(10)4-2-5/h1-4,8-9H/p-2
Benzalcyanoacetamides were designed and ...
The preparation of bicyclic pyrrolidines...
Myeloid cell leukemia 1 (Mcl-1) protein ...
Exploiting the redox sensitivity of disu...
The invention belongs to the field of sy...
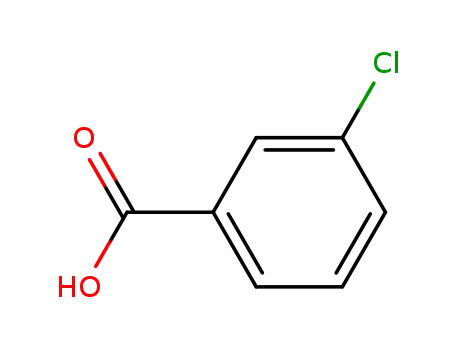
3-chlorobenzoate

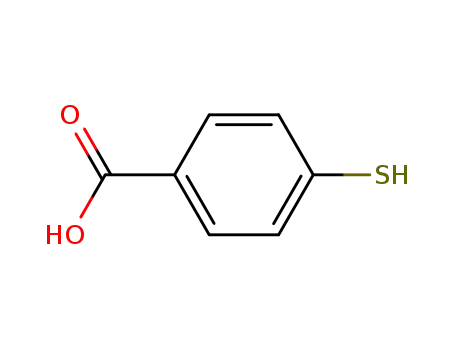
4-mercaptobenzoic acid

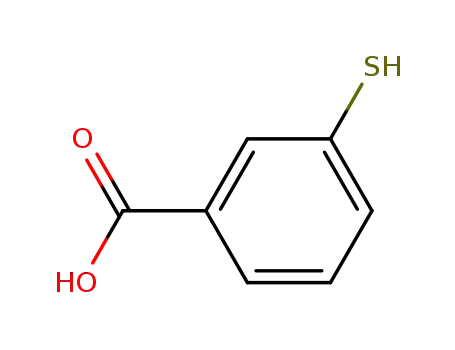
3-mercapto benzoic acid
| Conditions | Yield |
|---|---|
|
With sodium tetrahydroborate; sulfur; Yield given. Multistep reaction. Yields of byproduct given; 1.) 360 deg C, NaOH-KOH melt, 3 min.; 2.) water;
|
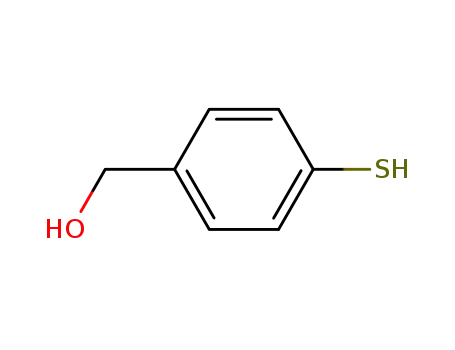
4-mercaptobenzyl alcohol


4-mercaptobenzoic acid
| Conditions | Yield |
|---|---|
|
With diethylene glycol dimethyl ether; at 70 ℃; for 0.5h; Sonication;
|
94% |
|
With oxygen; at 120 ℃; for 16h; Green chemistry;
|
84% |
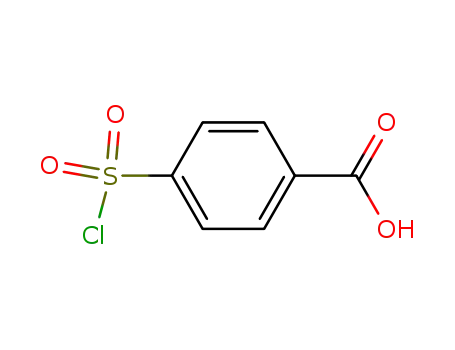
p-carboxyphenylsulfonyl chloride
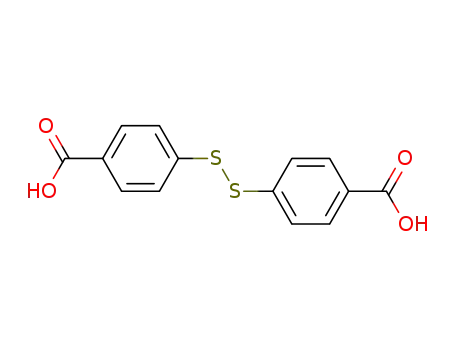
4,4'-Dithiobisbenzoic acid
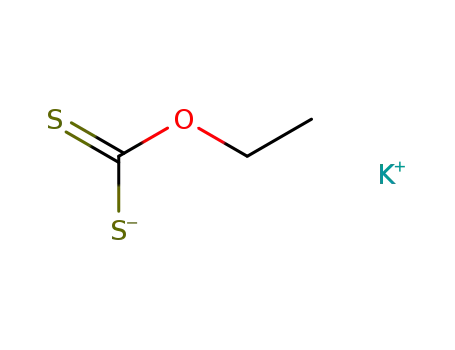
potassium ethyl xanthogenate
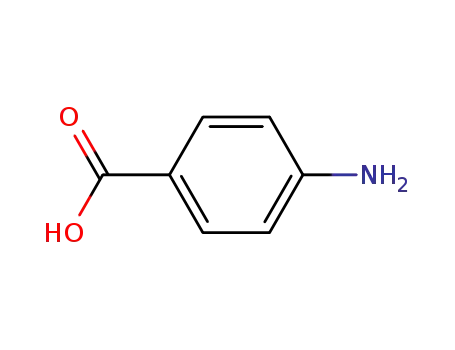
4-amino-benzoic acid
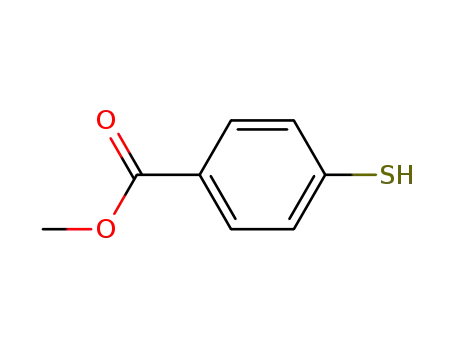
methyl 4-mercaptobenzoate
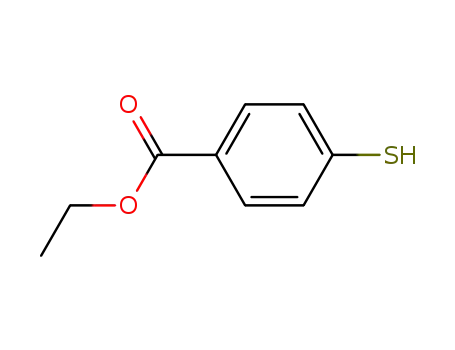
ethyl p-mercaptobenzoate

4,4'-Dithiobisbenzoic acid
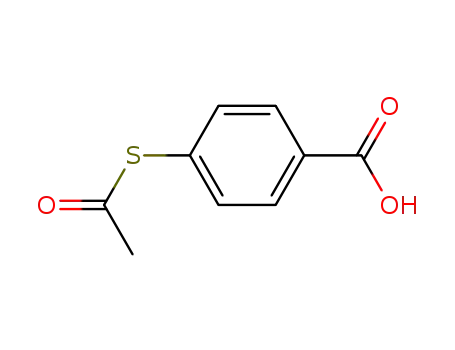
S-acetyl-4-mercaptobenzoic acid
CAS:14867-28-8
CAS:72-14-0
CAS:611-99-4
CAS:4224-69-5