Your Location:Home >Products >Intermediates >2309-49-1
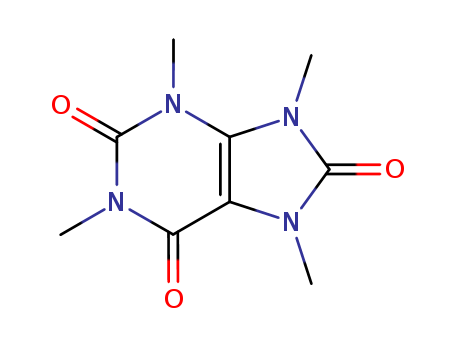

Product Details
|
Uses |
Tetramethyluric acid is an impurity of caffeine. Caffeine is a bitter, white crystalline xanthine alkaloid that acts as a stimulant drug and a reversible acetylcholinesterase inhibitor. Caffeine is found in varying quantities in the seeds, leaves, and fruit of some plants, where it acts as a natural pesticide that paralyzes and kills certain insects feeding on the plants. In humans, caffeine acts as a central nervous system stimulant, temporarily warding off drowsiness and restoring alertness. Caffeine is a cardiac and respiratory stimulant; diuretic. Caffeine is toxic at sufficiently high doses. |
|
Purification Methods |
Crystallise the uric acid from H2O or MeOH. [Beilstein 26 H 532, 26 I 156, 26 II 302, 21 III/IV 2623.] |
InChI:InChI=1/C9H12N4O3/c1-10-5-6(11(2)8(10)15)12(3)9(16)13(4)7(5)14/h1-4H3
9-Methylcaffeinium iodide, a bio-based s...
A one-pot direct synthesis of xanthine a...
The invention discloses a synthesis meth...
The invention provides preparation metho...
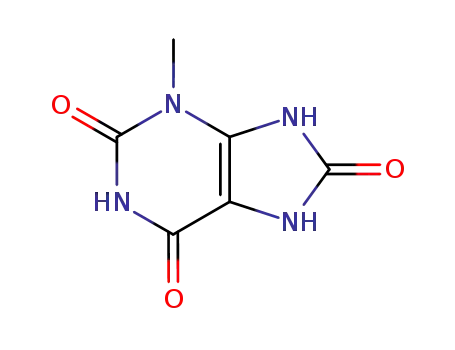
3-methyluric acid

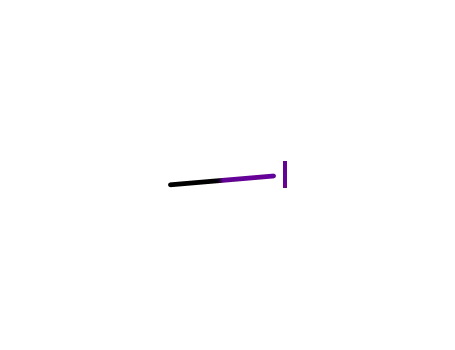
methyl iodide

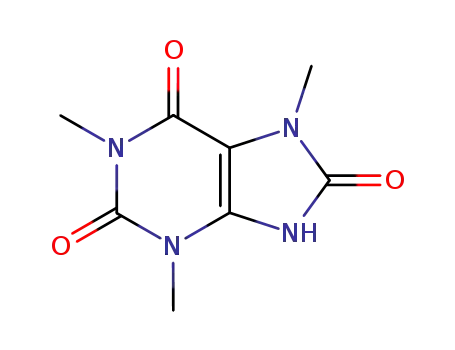
1,3,7-trimethyluric acid

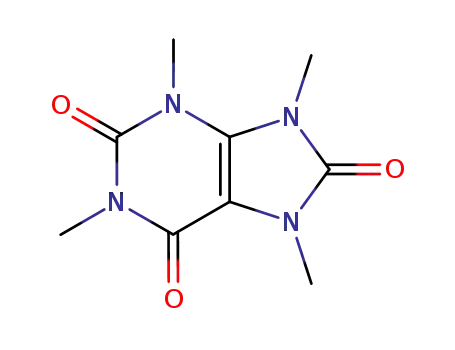
theacrine
| Conditions | Yield |
|---|---|
|
at 35 - 40 ℃;
|
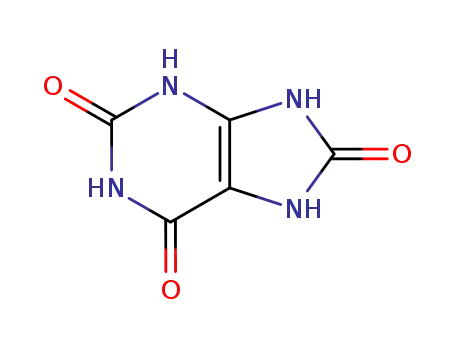
uric Acid


methyl iodide


1,3,7-trimethyluric acid


theacrine
| Conditions | Yield |
|---|---|
|
at 100 - 110 ℃; im ueberschuss;
|

diazomethane

uric Acid
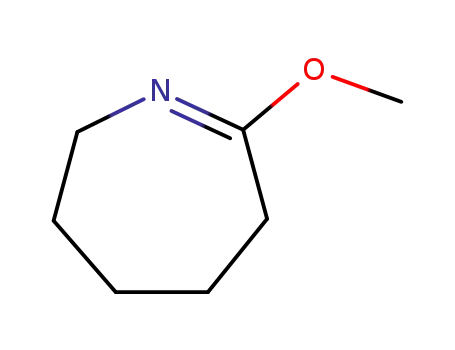
O-methylcaprolactim

methyl iodide
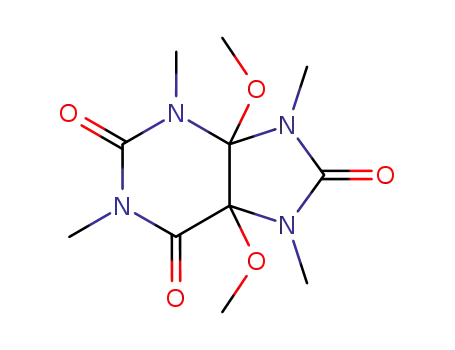
4,5-dimethoxy-1,3,7,9-tetramethyl-tetrahydro-purine-2,6,8-trione
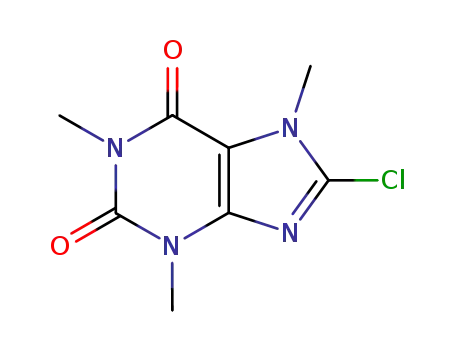
8-chlorocaffeine
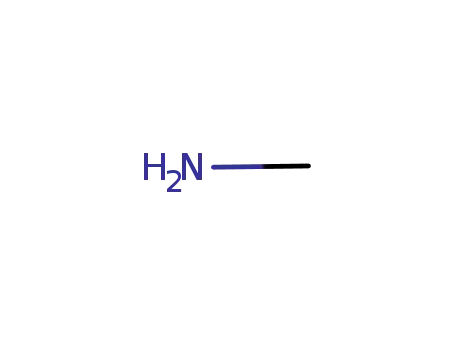
methylamine
CAS:2941-78-8
CAS:1143516-05-5
CAS:2935-90-2
CAS:51168-26-4