Your Location:Home >Products >Fine chemicals >541-59-3
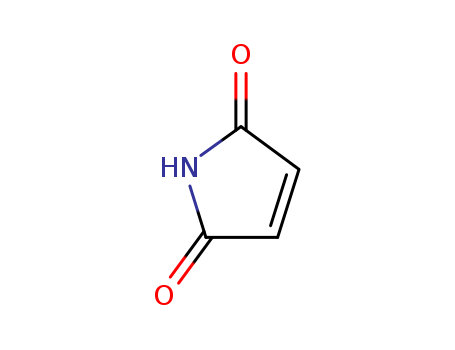

Product Details
|
Chemical Properties |
slight yellow crystalline powder |
|
Uses |
Rhodium-catalyzed conjugate arylation with arylboronic acids.1 |
|
Definition |
ChEBI: A cyclic dicarboximide in which the two carboacyl groups on nitrogen together with the nitogen itself form a 1H-pyrrole-2,5-dione structure. |
|
General Description |
Maleimide (2,5-Pyrroledione) is a new nanoparticle surface functional group which favors easy conjugation with cell penetration peptides. The conjugation is enabled via click chemistry to preserve its biofunctions. |
|
Hazard |
A poison. |
|
Safety Profile |
Poison by intraperitoneal and intravenous routes. An experimental teratogen. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx. |
|
Purification Methods |
Purify it by sublimation in a vacuum. The UV has max at 216 and 280nm in EtOH. [de Wolf & van de Straete Bull Soc Chim Belg 44 288 1935, UV: Rondestvedt et al. J Am Chem Soc 78 6115 1956, IR: Chiorboli & Mirone Ann Chim (Rome) 42 681 1952, Beilstein 21/10 V 3.] |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C4H3NO2/c6-3-1-2-4(7)5-3/h1-2H,(H,5,6,7)
The invention provides a preparation met...
PROBLEM TO BE SOLVED: To provide a metho...
Catalytic strategies were developed to s...
A novel protocol for ortho-C-H alkylatio...
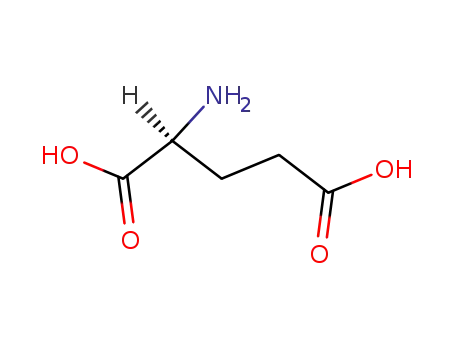
L-glutamic acid

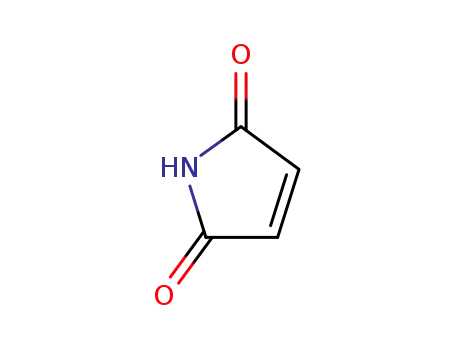
maleiimide

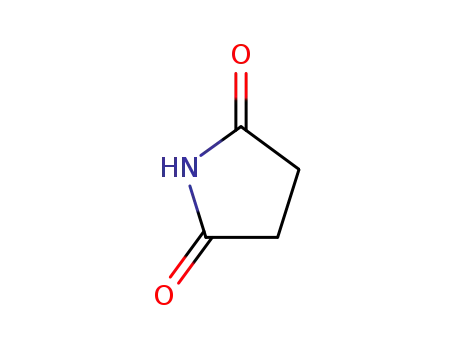
Succinimide

pyrrole

β-Propiolactone

3-Methylpyridine

ethanol

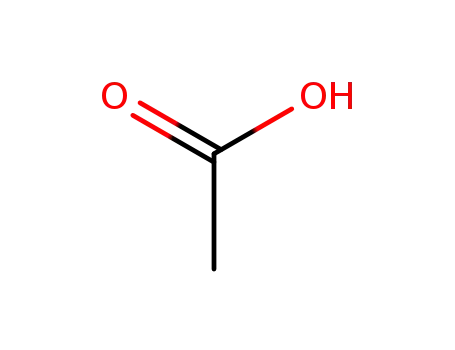
acetic acid

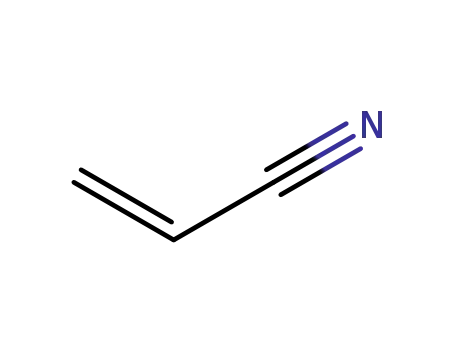
acrylonitrile

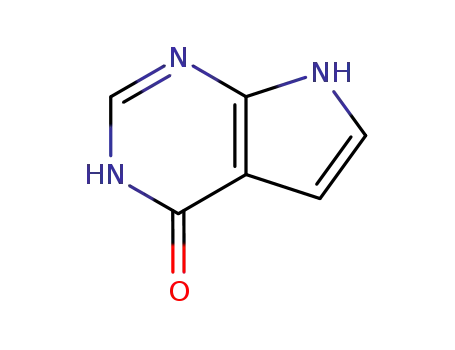
7-deazahypoxanthine


acetonitrile

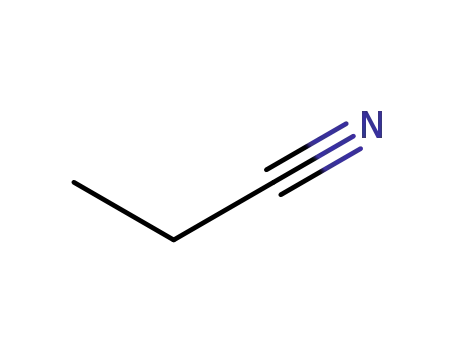
propiononitrile
| Conditions | Yield |
|---|---|
|
With oxygen; at 400 ℃; for 5.55556E-05h; under 760.051 Torr; Temperature; Inert atmosphere; Pyrolysis; Gas phase; Flow reactor;
|

L-glutamic acid

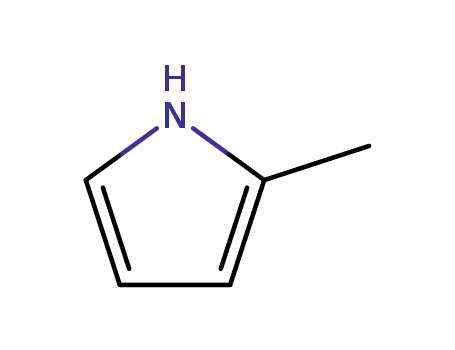
2-methyl-1H-pyrrole

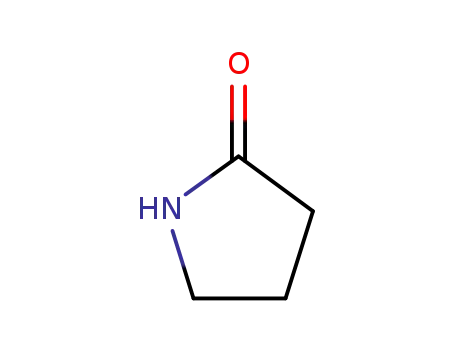
2-pyrrolidinon

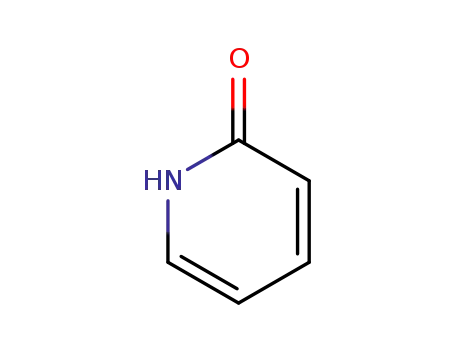
2-Pyridone


maleiimide


Succinimide


pyrrole


7-deazahypoxanthine


acetonitrile
| Conditions | Yield |
|---|---|
|
at 400 ℃; for 5.55556E-05h; under 760.051 Torr; Temperature; Inert atmosphere; Pyrolysis; Gas phase; Flow reactor;
|

Succinimide

pyrrole
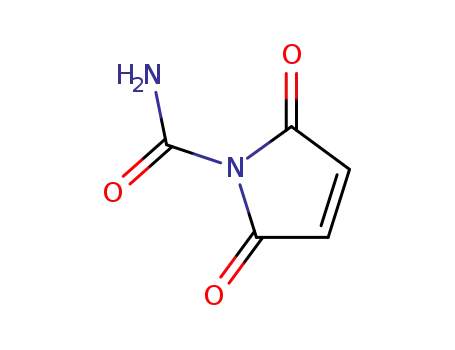
N-carbamoylmaleimide
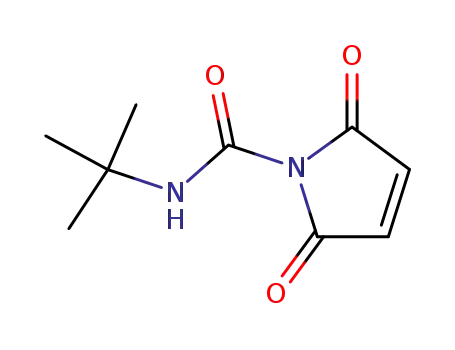
2,5-dioxo-2,5-dihydro-pyrrole-1-carboxylic acid tert-butylamide
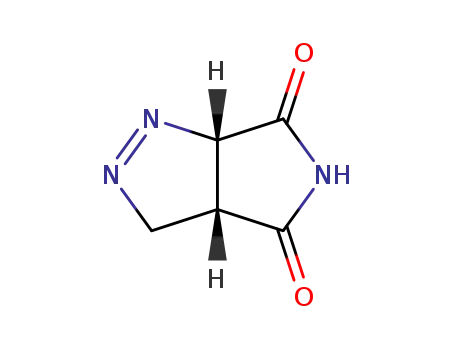
(+/-)-(3ar,6ac)-3a,6a-dihydro-3H-pyrrolo[3,4-c]pyrazole-4,6-dione
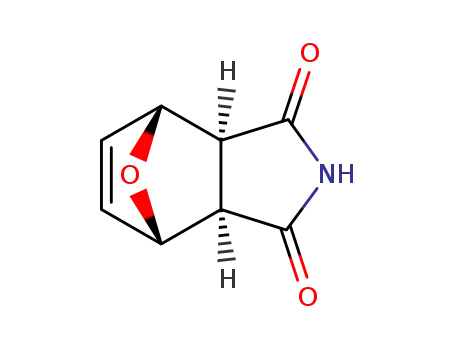
exo-7-oxabicyclo[2.2.1]hept-4-ene-2,3-dicarboximide
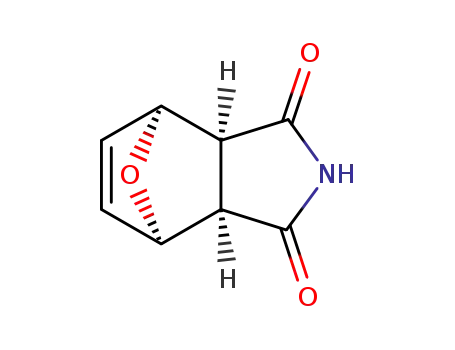
(3aR,4R,7S,7aS)-3a,4,7,7a-tetrahydro-1H-4,7-epoxyisoindole-1,3(2H)-dione
3-(phenylamino)pyrrolidine-2,5-dione
CAS:2941-78-8
CAS:1143516-05-5
CAS:60-56-0
CAS:1750-42-1