Your Location:Home >Products >Fine chemicals >16184-89-7
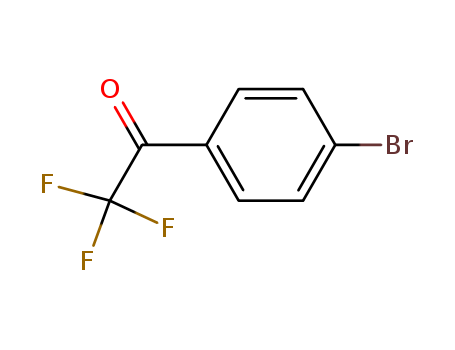

Product Details
|
Chemical Properties |
Clear light yellow liquid |
|
Uses |
4?-Bromo-2,2,2-trifluoroacetophenone may be used in the preparation of carbonyl-bridged bithiazole derivatives. Also used as a reagent to synthesize MK-5046, a selective Bombesin receptor subtype-3 agonist used to treat obesity. |
InChI:InChI=1/C10H9F3O/c1-2-9(14)7-5-3-4-6-8(7)10(11,12)13/h3-6H,2H2,1H3
The novel vinylogous aldol-lactonization...
A palladium-catalyzed carbonylative coup...
Alkenes, ethers, and alcohols account fo...
A novel class of chiral spiro-fused biso...
A straightforward method that enables th...
A simple and straightforward approach to...
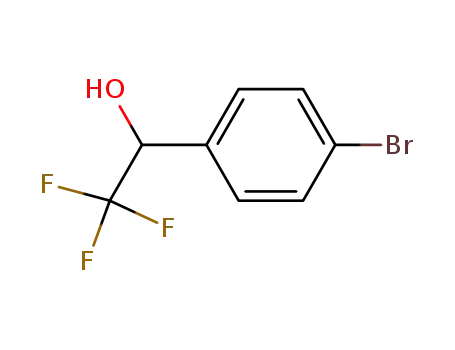
1-(4-bromo-phenyl)-2,2,2-trifluoro-ethanol

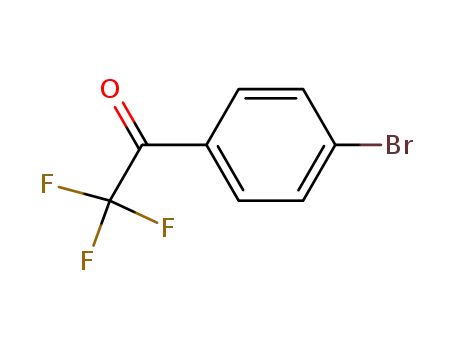
4'-bromo-2,2,2-trifluoroacetophenone
| Conditions | Yield |
|---|---|
|
With 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione; In ethyl acetate; for 20h; Reflux;
|
96% |
|
1-(4-bromo-phenyl)-2,2,2-trifluoro-ethanol; With N-(2,2,6,6-tetramethyl-1-oxopiperidin-1-ium-4-yl)acetamide tetrafluoroborate; In dichloromethane; at 20 ℃; for 0.0833333h;
With 2,6-dimethylpyridine; In dichloromethane; at 20 ℃;
|
74% |
|
With 9-azabicyclo[3.3.1]nonan-3-one N-oxyl oxide; oxygen; acetic acid; sodium nitrite; at 20 ℃; for 18h; under 760.051 Torr; chemoselective reaction;
|
65% |
|
With tetra(n-butyl)ammonium hydrogen sulfate; In dichloromethane; water;
|
|
|
With 1,10-Phenanthroline; diethyl hydrazodicarboxylate; oxygen; potassium carbonate; copper(l) chloride; In toluene; at 90 ℃;
|
|
|
With dipyridinium dichromate; In dichloromethane; for 18h; Reflux; Inert atmosphere;
|
|
|
With sodium carbonate; Dess-Martin periodane; In dichloromethane; at 20 ℃; for 3h;
|
|
|
In dichloromethane; Reflux; Inert atmosphere;
|
795 mg |
|
With 2,6-dimethylpyridine; N-(2,2,6,6-tetramethyl-1-oxopiperidin-1-ium-4-yl)acetamide tetrafluoroborate; In dichloromethane; at 20 ℃; for 12h; Inert atmosphere; Schlenk technique;
|
|
|
With sodium hydrogencarbonate; Dess-Martin periodane; In dichloromethane; at 0 - 20 ℃; for 12h;
|
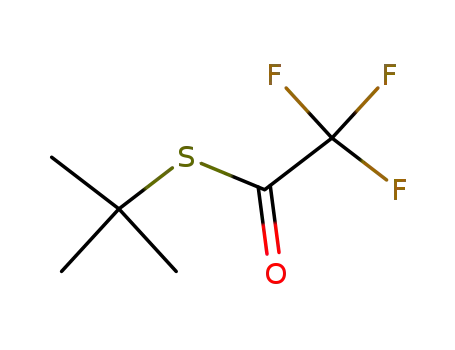
S-(tert-butyl)trifluorothioacetate

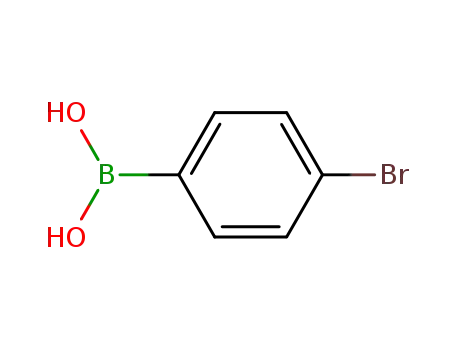
4-Bromophenylboronic acid


4'-bromo-2,2,2-trifluoroacetophenone
| Conditions | Yield |
|---|---|
|
With tris-(dibenzylideneacetone)dipalladium(0); copper(I) thiophene-2-carboxylate; trifuran-2-yl-phosphane; In tetrahydrofuran; at 30 ℃; for 18h; Sealed tube; Inert atmosphere;
|
67% |
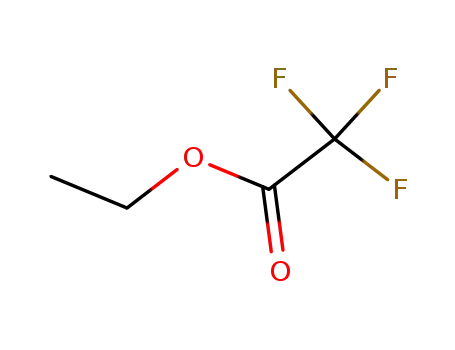
ethyl trifluoroacetate,
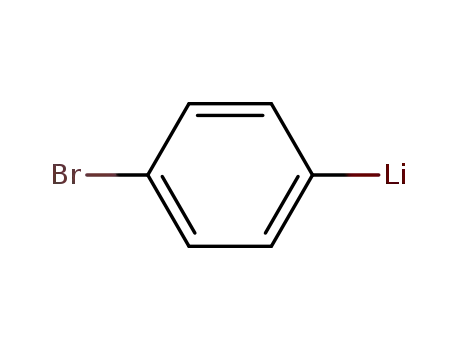
4-bromophenyllithium
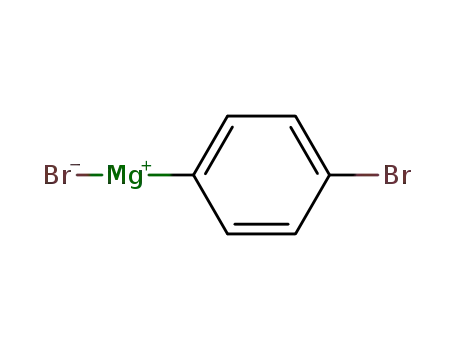
(4-bromophenyl)magnesium bromide
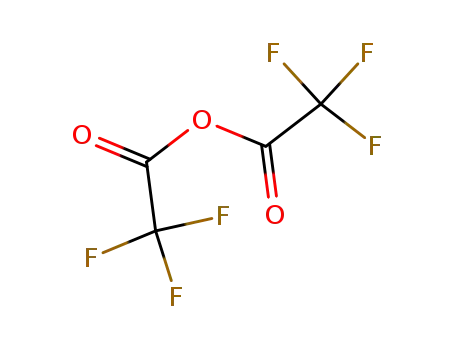
trifluoroacetic anhydride
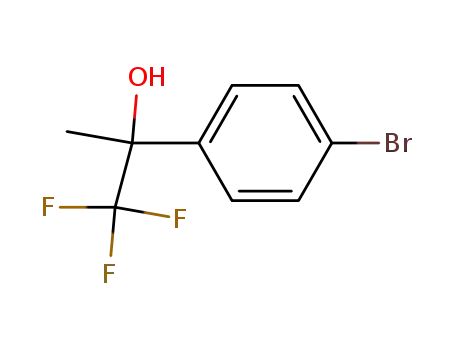
2‐(4‐bromophenyl)‐1,1,1‐trifluoropropan‐2‐ol

1-(4-bromo-phenyl)-2,2,2-trifluoro-ethanol
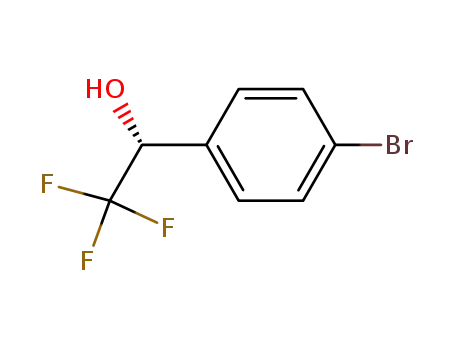
(R)-1-(4-bromophenyl)-2,2,2-trifluoroethanol
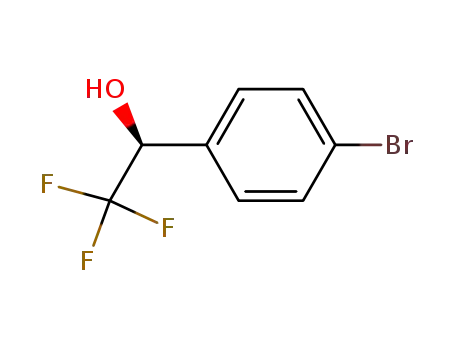
(S)-1-(4-bromophenyl)-2,2,2-trifluoroethan-1-ol
CAS:928715-37-1
CAS:6482-24-2
CAS:622-47-9
CAS:1072-67-9