Your Location:Home >Products >Intermediates >477600-74-1

Product Details
|
Uses |
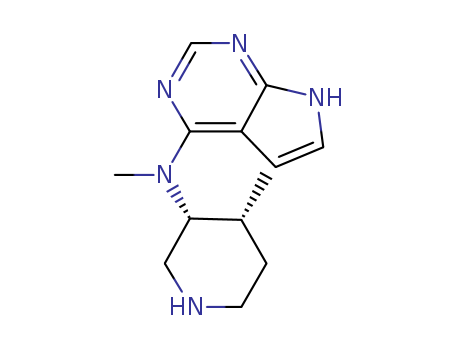
N-Methyl-N-((3S,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine, is an intermediate in the synthesis of (3S,4R)-Tofacitinib (T528010), an enantiopure stereoisomer of the drug, Janus kinase 3(Jak3) inhibitor (CP-690,550) that has been found to inhibit selected members of the STE7 and STE20 subfamily of kinases. |
InChI:InChI=1/C13H19N5/c1-9-3-5-14-7-11(9)18(2)13-10-4-6-15-12(10)16-8-17-13/h4,6,8-9,11,14H,3,5,7H2,1-2H3,(H,15,16,17)/t9-,11+/m1/s1
An enantioselective total synthesis of T...
Provided is a process for the preparatio...
The invention discloses a tofacitinib ci...
The invention discloses a preparation me...
![((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-(7H-pyrrolo[2,3-d]pyrimidine-4-yl)amine](/upload/2024/4/162edbe1-1957-4469-8299-58139da5b4fa.png)
((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-(7H-pyrrolo[2,3-d]pyrimidine-4-yl)amine

![tert-butyl (3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate](/upload/2024/4/eddafd50-eac6-4b39-a6e8-ea37e4e0adf2.png)
tert-butyl (3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate
| Conditions | Yield |
|---|---|
|
With palladium 10% on activated carbon; 20% palladium hydroxide-activated charcoal; hydrogen; In methanol; at 45 ℃; under 6000.6 Torr;
|
93% |
|
With 20% palladium hydroxide-activated charcoal; ammonium formate; In methanol; at 60 ℃; for 6h; Solvent; Reagent/catalyst; Temperature; Inert atmosphere;
|
93.3% |
|
With 20% palladium hydroxide-activated charcoal; hydrogen; acetic acid; In methanol; water; at 50 ℃; for 12h; under 3000.3 - 4500.45 Torr;
|
92.38% |
|
With hydrogenchloride; hydrogen; palladium(II) hydroxide/carbon; In ethanol; water; at 20 ℃; for 48h; under 2585.81 Torr;
|
90% |
|
((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-(7H-pyrrolo[2,3-d]pyrimidine-4-yl)amine; With hydrogen; acetic acid; 20% Pd(OH)2 on carbon; In water; isopropyl alcohol; at 45 - 55 ℃; under 2585.81 Torr;
With sodium hydroxide; water; at 75 - 90 ℃; for 1h; Product distribution / selectivity;
|
87.3% |
|
With palladium 10% on activated carbon; hydrogen; ammonium formate; In ethanol; at 80 ℃; Temperature; Reagent/catalyst;
|
83.6% |
|
With formic acid; palladium 10% on activated carbon; In methanol; at 67 - 70 ℃; Reagent/catalyst; Temperature;
|
82.4% |
|
((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-(7H-pyrrolo[2,3-d]pyrimidine-4-yl)amine; With hydrogen; acetic acid; palladium hydroxide on carbon; In water; isopropyl alcohol; at 50 ℃; for 16h;
With sodium hydroxide; In water; isopropyl alcohol; pH=8;
|
81% |
|
With palladium 10% on activated carbon; hydrogen; acetic acid; In water; isopropyl alcohol; at 50 ℃; for 16h;
|
74% |
|
With hydrogen; trifluoroacetic acid; palladium(II) hydroxide; In methanol; for 5.5h; under 2585.81 Torr; in a Parr shaker;
|
39% |
|
With Pd(OH)2/C; hydrogen; trifluoroacetic acid; In methanol; for 6h; under 2585.81 Torr;
|
|
|
With 10 wt% Pd(OH)2 on carbon; hydrogen; In methanol; acetic acid; at 50 ℃; for 6h; under 760.051 Torr;
|
|
|
With hydrogen; palladium(II) hydroxide; acetic acid; In water; isopropyl alcohol; at 25 - 30 ℃; for 8h; under 2250.23 - 3000.3 Torr; Temperature; Inert atmosphere; Large scale;
|
1.24 kg |
|
With 20% palladium hydroxide-activated charcoal; hydrogen; trifluoroacetic acid; In methanol; for 5h; under 3620.13 - 4137.29 Torr;
|
|
|
With palladium 10% on activated carbon; ammonium formate; In methanol; for 2h; Concentration; Reflux;
|
|
|
Multi-step reaction with 2 steps
1: palladium 10% on activated carbon / methanol / 2 h / 20 - 70 °C
2: potassium carbonate / dichloromethane; water / 1 h / 20 °C
With palladium 10% on activated carbon; potassium carbonate; In methanol; dichloromethane; water;
|
|
|
With palladium 10% on activated carbon; hydrogen; In methanol; for 24h;
|
0.97% |
|
With 20% palladium hydroxide-activated charcoal; hydrogen; acetic acid; In water;
|
77 g |
|
With palladium 10% on activated carbon; hydrogen; In methanol; at 20 ℃;
|
6 g |
|
With palladium 10% on activated carbon; hydrogen; Trimethylacetic acid; In water; at 45 ℃; Reagent/catalyst; Temperature; Solvent; Catalytic behavior;
|
|
|
With palladium on carbon; hydrogen; trifluoroacetic acid; In methanol; at 45 ℃; for 12h; under 760.051 Torr;
|
|
|
((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-(7H-pyrrolo[2,3-d]pyrimidine-4-yl)amine; With hydrogenchloride; In water; at 20 ℃; pH=3.5 - 4.5;
With 5%-palladium/activated carbon; hydrogen; at 45 - 50 ℃;
|
|
|
With palladium 10% on activated carbon; hydrogen; acetic acid; In water; at 50 ℃; for 8h; Temperature;
|
![(3R,4R)-(1-benzyl-4-methylpiperidin-3-yl)-2-chloro-N-methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amine](/upload/2024/4/4312580d-40b2-49e0-9dd7-e76885a21bda.png)
(3R,4R)-(1-benzyl-4-methylpiperidin-3-yl)-2-chloro-N-methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amine

![tert-butyl (3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate](/upload/2024/4/eddafd50-eac6-4b39-a6e8-ea37e4e0adf2.png)
tert-butyl (3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; palladium 10% on activated carbon; hydrogen; In water; at 65 - 75 ℃; for 3h; under 1551.49 Torr; Inert atmosphere;
|
96% |
|
With triethylsilane; 5%-palladium/activated carbon; acetic acid; In methanol; at 30 ℃; for 0.166667h;
|
93.1% |
|
With 10 wt% Pd(OH)2 on carbon; hydrazine hydrate; acetic acid; In ethanol; at 70 - 75 ℃; for 2h; Reagent/catalyst;
|
75.8% |
|
With hydrogen; 20% Pd(OH)2 on carbon; In water; at 70 - 75 ℃; for 1h; under 2585.81 Torr; Product distribution / selectivity;
|
|
|
With hydrogenchloride; palladium on carbon; hydrogen; In water; at 120 ℃; under 2068.65 Torr; Reagent/catalyst; Solvent; Temperature; Pressure;
|
|
|
With palladium 10% on activated carbon; hydrogen; acetic acid; In ethanol; water; at 40 ℃; for 4h; under 900.09 Torr; Temperature; Pressure;
|
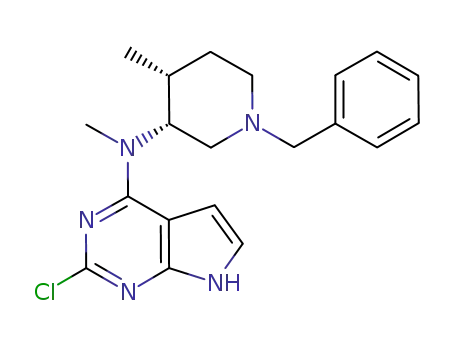
(3R,4R)-(1-benzyl-4-methylpiperidin-3-yl)-2-chloro-N-methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amine
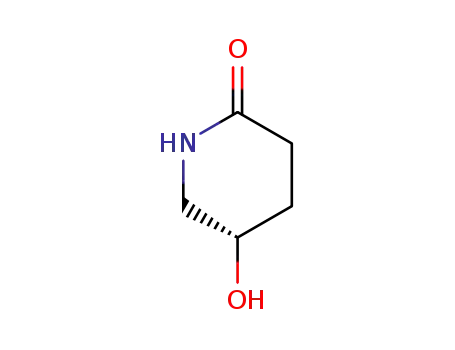
(S)-5-hydroxypiperidin-2-one
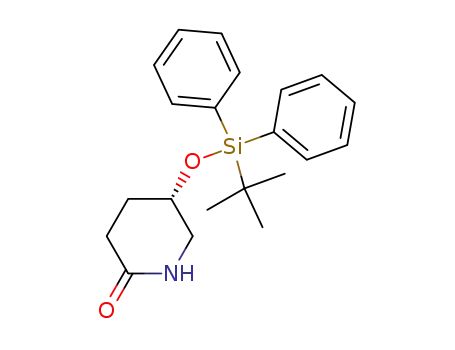
(5S)-5-tert-butyldiphenylsilyloxy-piperidine-2-one
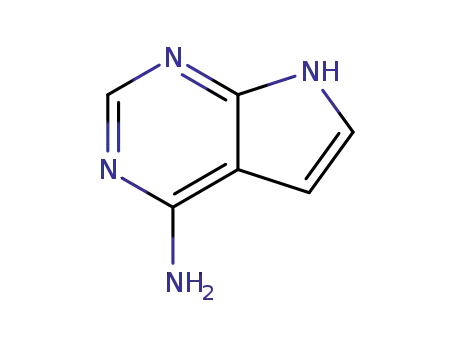
7-deazaadenine
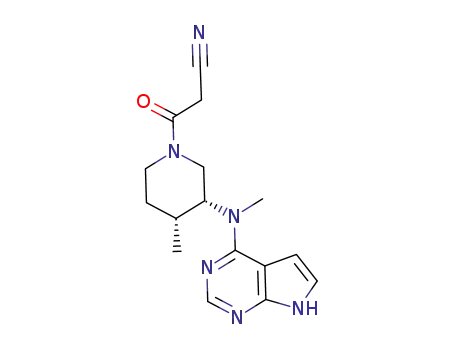
tasocitinib
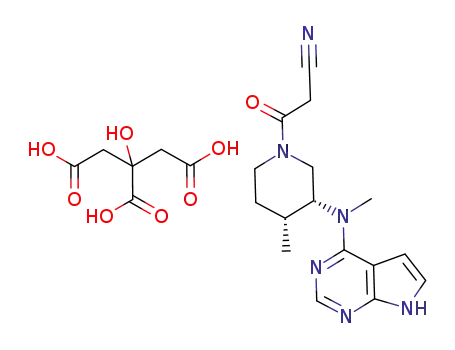
Tofacitinib citrate
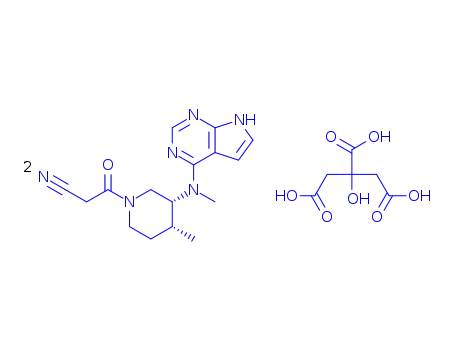
tofacitinib hemi-citrate
C18H25N5O3
CAS:2941-78-8
CAS:1143516-05-5
CAS:367-93-1