Your Location:Home >Products >API >122547-49-3
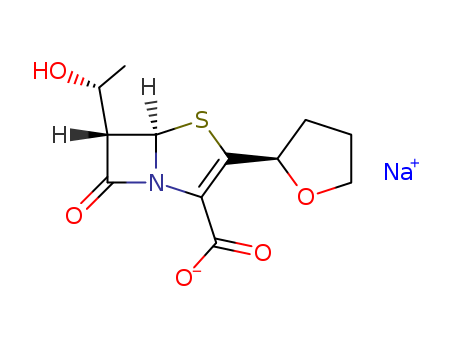

Product Details
|
Chemical Properties |
Pale Yellow Crystals |
|
Uses |
Faropenem is an orally active beta-lactam antibiotic belonging to the penem group. |
|
General Description |
Faropenem sodium hydrate belongs to the penem group of antibiotics prescribed for oral usage. Enterobacteriaceae bacterial infections with cephalosporin resistance are susceptible to faropenem. Faropenem could be an effective antibiotic to treat urinary tract infections caused by extended-spectrum beta-lactamases (ESBL) producing bacteria. |
|
Biochem/physiol Actions |
Faropenem sodium is an ultra-broad spectrum, β-lactamase resistant, β-lactam antibiotic active against both Gram-positive and Gram-negative bacteria. |
InChI:InChI=1/C12H15NO5S.Na/c1-5(14)7-10(15)13-8(12(16)17)9(19-11(7)13)6-3-2-4-18-6;/h5-7,11,14H,2-4H2,1H3,(H,16,17);/q;+1/p-1/t5-,6-,7+,11-;/m1./s1
An improved palladium(II)-catalyzed clea...
The intermediate is easy to prepare, low...
The invention discloses a preparation me...
The invention provides a method for synt...
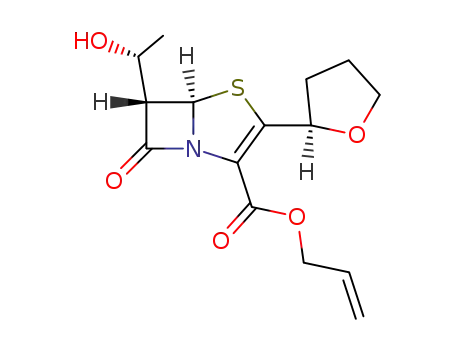
(5R,6S)-6-((R)-1-hydroxyethyl)-2-((R)-tetrahydro-2-furyl)penem-3-carboxylic acid allyl ester

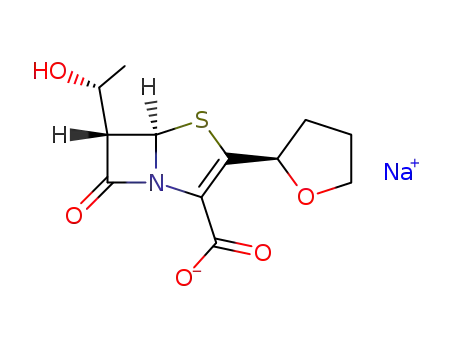
Furopenem
| Conditions | Yield |
|---|---|
|
With bis-triphenylphosphine-palladium(II) chloride; triphenylphosphine; sodium 2-ethylhexanoic acid; In water; ethyl acetate; at 20 ℃; for 5h; Inert atmosphere; Large scale reaction;
|
86.5% |
|
(5R,6S)-6-((R)-1-hydroxyethyl)-2-((R)-tetrahydro-2-furyl)penem-3-carboxylic acid allyl ester; With sodium isooctanoate; sodium caprylate; triphenylphosphine; In dichloromethane; ethyl acetate; at 20 ℃;
With tetrakis(triphenylphosphine) palladium(0); In dichloromethane; ethyl acetate; at 25 - 30 ℃; for 0.666667h; Inert atmosphere;
|
73.3% |
|
With tetrakis(triphenylphosphine) palladium(0); triphenylphosphine; sodium 2-ethylhexanoic acid; In ethyl acetate; for 1h; Ambient temperature;
|
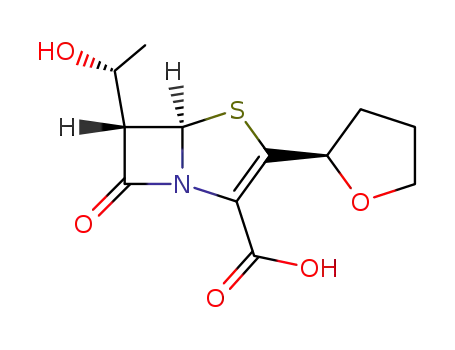
faropenem


Furopenem
| Conditions | Yield |
|---|---|
|
With sodium 2-ethylhexanoic acid; In tetrahydrofuran; water; at 20 ℃; for 2h;
|
125 g |

(5R,6S)-6-((R)-1-hydroxyethyl)-2-((R)-tetrahydro-2-furyl)penem-3-carboxylic acid allyl ester
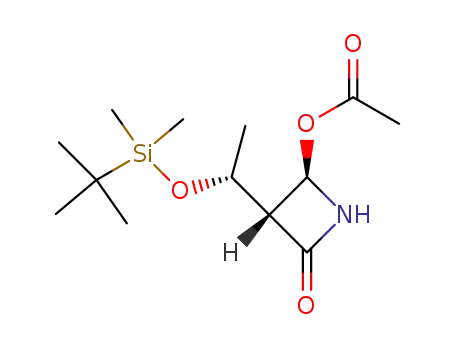
(3R,4R)-3-[(R)-1-(tert-butyldimethylsilyloxy)ethyl]-4-acetoxyazetidin-2-one
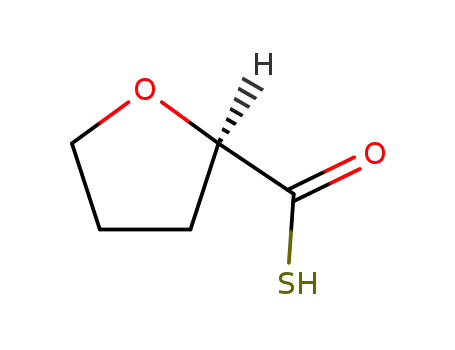
R-(+)-thio tetrahydrofuran-2-carboxylic acid
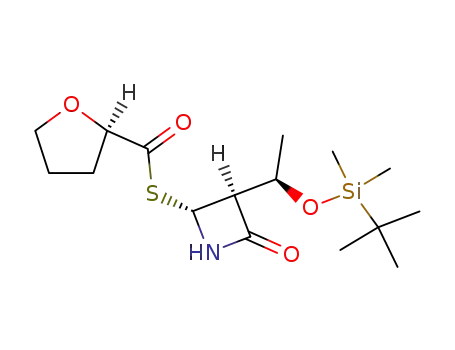
(R)-Tetrahydro-furan-2-carbothioic acid S-{(2R,3S)-3-[(R)-1-(tert-butyl-dimethyl-silanyloxy)-ethyl]-4-oxo-azetidin-2-yl} ester
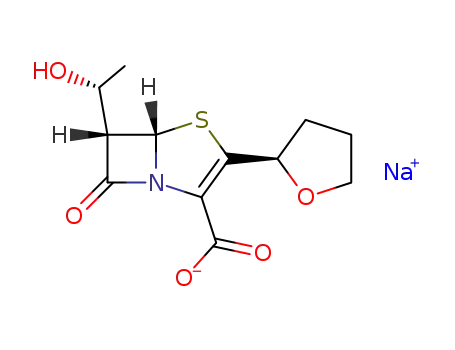
Sodium; (5S,6S)-6-((R)-1-hydroxy-ethyl)-7-oxo-3-(R)-tetrahydro-furan-2-yl-4-thia-1-aza-bicyclo[3.2.0]hept-2-ene-2-carboxylate
CAS:62-33-9
CAS:78415-72-2
CAS:69304-47-8