Your Location:Home >Products >API >69304-47-8
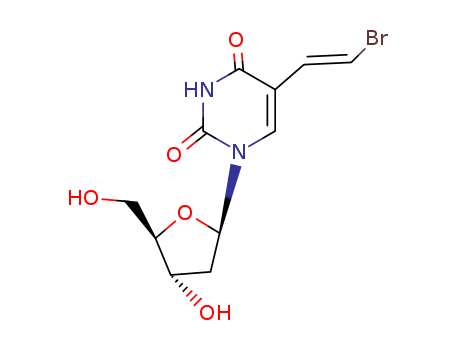

Product Details
|
Description |
(E)-5-(2-Bromovinyl)-2'-deoxyuridine is an thymidine analogue and acts as an anti-viral by inhibiting DNA plymerase. |
|
Chemical Properties |
White solid |
|
Uses |
It is used as pharmaceutical intermediate. |
InChI:InChI=1/C13H17Cl3N4.2ClH/c1-20(5-2-14)8-9-6-10(15)12(11(16)7-9)19-13-17-3-4-18-13;;/h6-7H,2-5,8H2,1H3,(H2,17,18,19);2*1H
Syntheses of 5-(2-[18F]fluoroethyl)- (1)...
Pd-imidate complexes have been employed ...
The invention discloses a novel synthesi...
Nucleosides represent a major chemothera...
Pyrimidine nucleoside phosphorylase from...
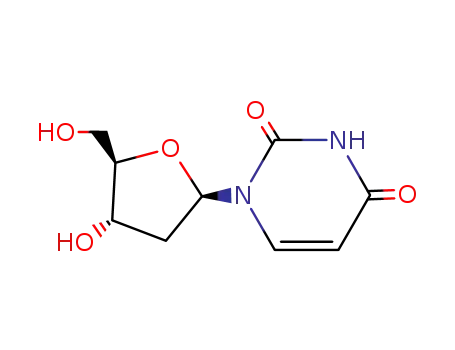
2'-deoxyuridine

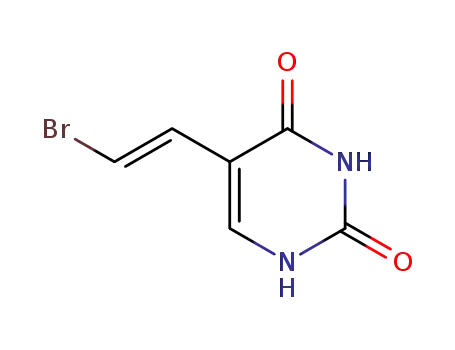
(E)-5-(2-bromovinyl)uracil

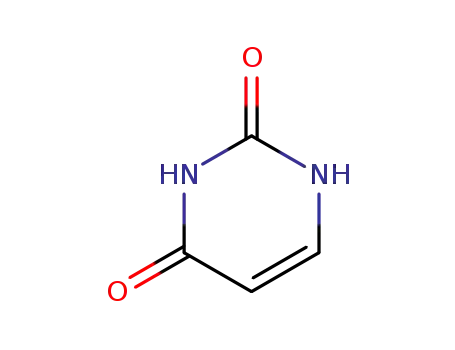
uracil

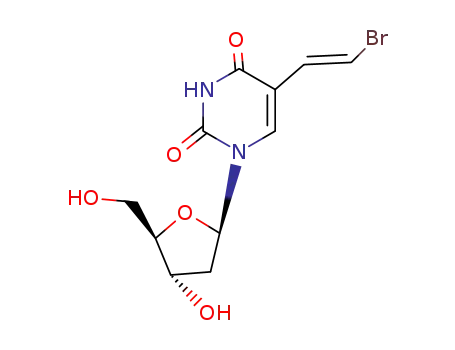
(E)-5-(2-bromovinyl)-2'-deoxyuridine
| Conditions | Yield |
|---|---|
|
With potassium carbonate; In aq. phosphate buffer; at 20 ℃; for 3h; pH=10; Reagent/catalyst; Enzymatic reaction;
|
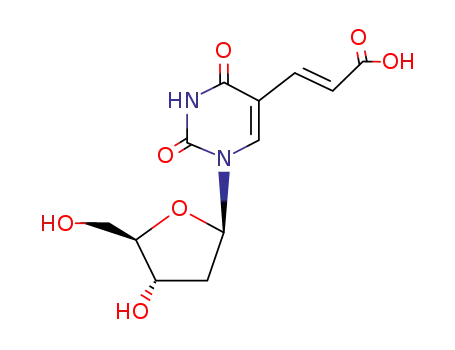
(E)-3-(1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)acrylic acid


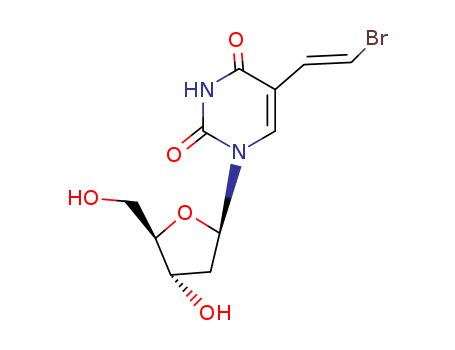
(E)-5-(2-bromovinyl)-2'-deoxyuridine
| Conditions | Yield |
|---|---|
|
With N-Bromosuccinimide; In tetrahydrofuran; water; at 25 ℃; for 0.5h;
|
95% |
|
(E)-3-(1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)acrylic acid; With potassium carbonate; In N,N-dimethyl-formamide; at 20 ℃; for 0.25h;
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃; for 0.5h;
|
89% |
|
(E)-3-(1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)acrylic acid; With potassium carbonate; In DMF (N,N-dimethyl-formamide); at 20 ℃; for 0.25h;
With N-Bromosuccinimide; In DMF (N,N-dimethyl-formamide); at 20 ℃; for 0.5h;
|
71.9% |
|
With N-Bromosuccinimide; 1-ethylene glycol monomethyl ether-3-methylimidazolium methanesulfonate; at 20 ℃; for 1h;
|
70% |
|
With N-Bromosuccinimide; potassium carbonate; In N,N-dimethyl-formamide;
|
150 mg (67%) |
|
With N-Bromosuccinimide; potassium carbonate;
|
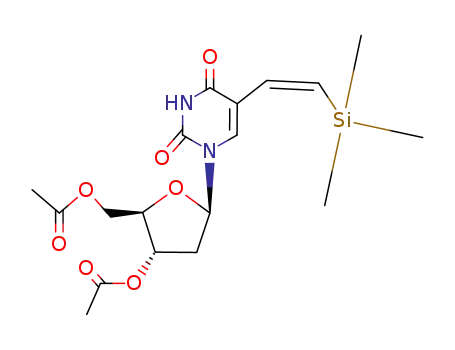
3',5'-Di-O-acetyl-(Z)-5-<2-(trimethylsilyl)ethenyl>-2'-deoxyuridine
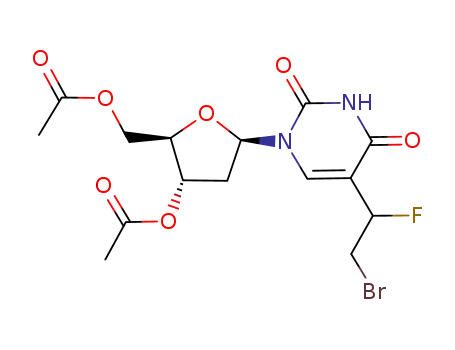
5-(1-fluoro-2-bromoethyl)-3',5'-di-O-acetyl-2'-deoxyuridine
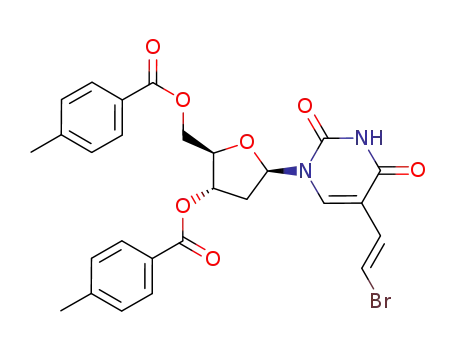
(E)-5-(2-bromovinyl)-2'-deoxy-3',5'-di-O-(p-toluoyl)uridine

(E)-3-(1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)acrylic acid
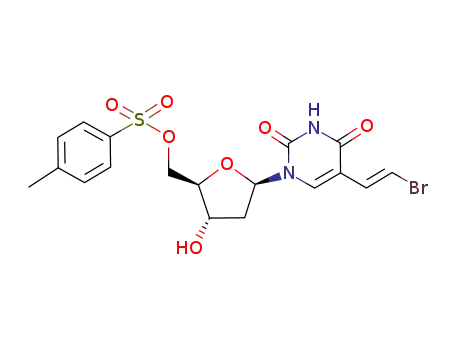
(E)-5-(2-bromovinyl)-2'-deoxy-5'-O-p-toluenesulphonyluridine
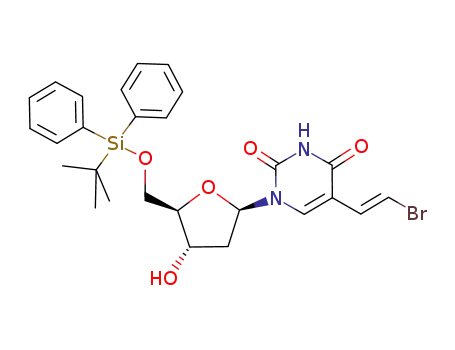
(E)-5-(2-bromovinyl)-2'-deoxy-5'-O-t-butyldiphenylsilyluridine
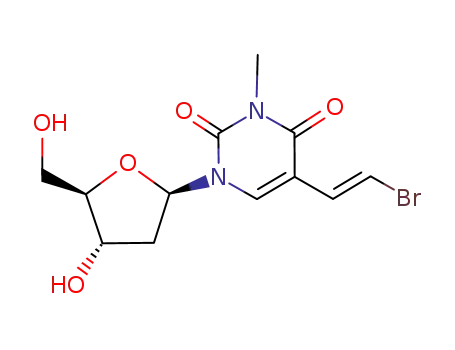
(E)-5-(2-bromovinyl)-2'-deoxy-3-methyluridine
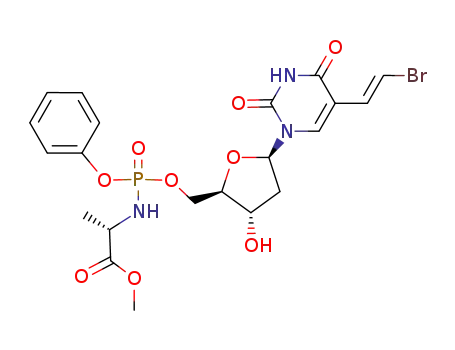
(E)-5-(2-bromovinyl)-2'-deoxy-5'-uridyl phenyl L-methoxyalaninylphosphoramidate
CAS:114-49-8
CAS:300-08-3
CAS:122547-49-3
CAS:28319-77-9