Your Location:Home >Products >Intermediates >17422-32-1
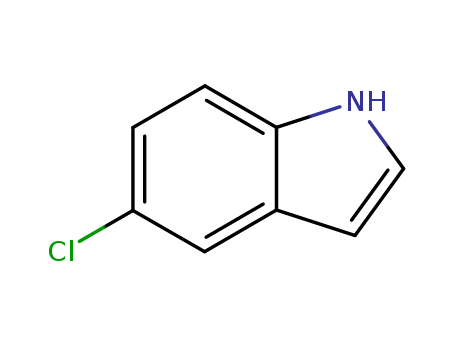

Product Details
|
Chemical Properties |
White to slightly greyish-green crystalline powder |
|
Uses |
5-Chloroindole is a halo-substituted indole used in the preparation of neurologically active compounds such as atypical antipsychotic agents. 5-Chloroindole has been shown to depress serotonin levels in the brainstem and telencephalon. |
|
Synthesis Reference(s) |
Journal of Heterocyclic Chemistry, 29, p. 1625, 1992 DOI: 10.1002/jhet.5570290644The Journal of Organic Chemistry, 44, p. 578, 1979 DOI: 10.1021/jo01318a021 |
|
General Description |
5-Chloroindole is a 5-substituted indole. It undergoes electropolymerization to form a redox-active film consisting of a cyclic trimer and chains of linked cyclic trimer (polymer). It is a potential positive allosteric modulator (PAM) of the 5-HT3 receptor. It has been reported as strong inhibitor of the copper dissolution in acidic sodium chloride solution. It has been tested as corrosion inhibitor of mild steel in 1N deaerated sulphuric acid. Synthesis of 5-chloroindole, via nitration of indoline has been described. |
|
Purification Methods |
It is distilled at high vacuum and recrystallises from pet ether (b 40-60o) or (b 80-100o) as glistening plates. The picrate has m 147o (146.5-147.5o)(from *C6H6). [Rydon & Tweddle J Chem Soc 3499 1955, Sugasawa J Org Chem 44 578 1979, Beilstein 20/4 V 34.] |
InChI:InChI=1/C8H6ClN/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H
We report the design of a bifunctional m...
N-heterocycles are key structures for ma...
A NaH-mediated detosylation reaction of ...
Herein, we report a protocol for desulfo...
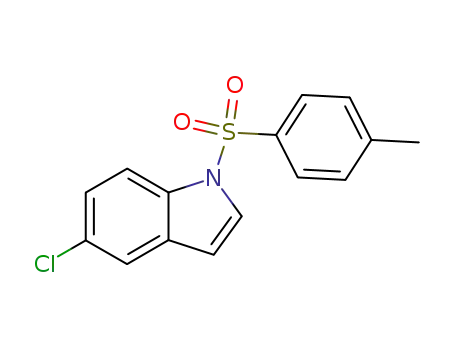
5-chloro-1-p-toluenesulfonylindole

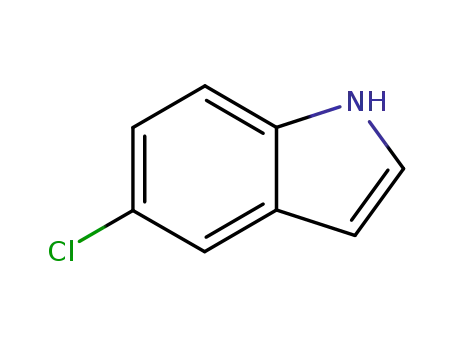
5-chloro-1H-indole
| Conditions | Yield |
|---|---|
|
With sodium hydride; In N,N-dimethyl acetamide; at 60 ℃; for 3h; Inert atmosphere;
|
92% |
|
With formic acid; (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; N-ethyl-N,N-diisopropylamine; In acetonitrile; at 20 ℃; for 24h; Inert atmosphere; Sealed tube; Irradiation;
|
89% |
|
With sodium hydroxide; In methanol; for 12h; Heating;
|
75% |
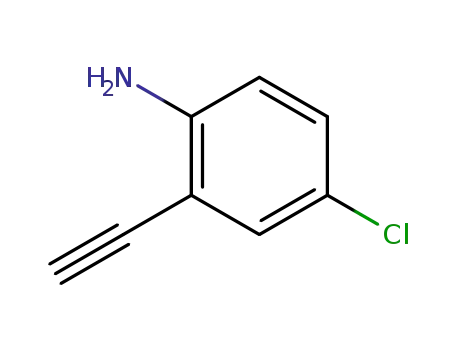
4-chloro-2-ethynylaniline


5-chloro-1H-indole
| Conditions | Yield |
|---|---|
|
With P(p-C6H4F)3; chloro(1,5-cyclooctadiene)rhodium(I) dimer; In N,N-dimethyl-formamide; at 85 ℃; for 2h;
|
88% |
|
With pyrrolidine; In water; at 200 ℃; for 0.25h; Microwave irradiation;
|
86% |
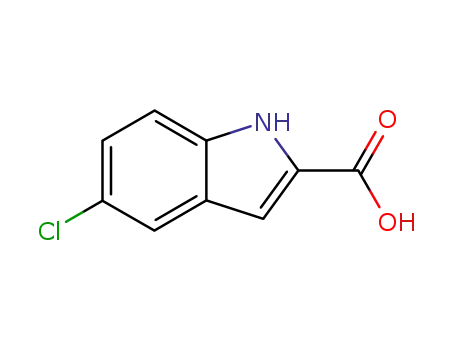
5-chloro-1H-Indole-2-carboxylic acid
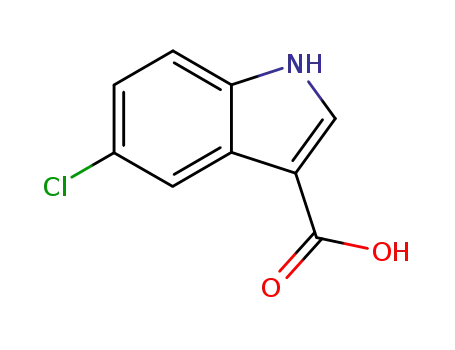
5-chloro-1H-indole-3-carboxylic acid
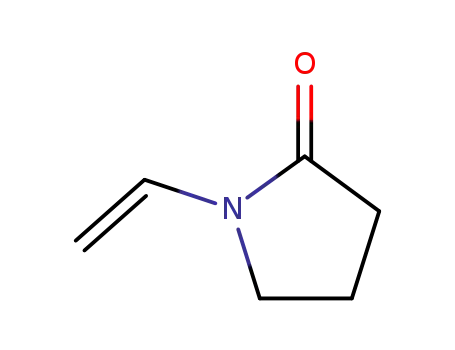
1-ethenyl-2-pyrrolidinone
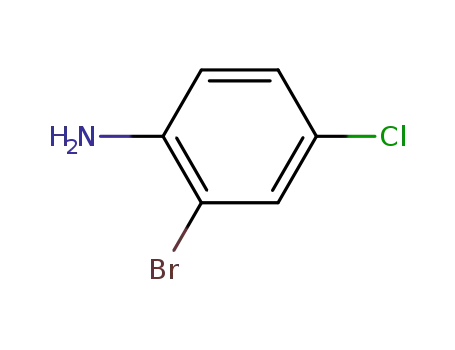
4-chloro-2-bromoaniline
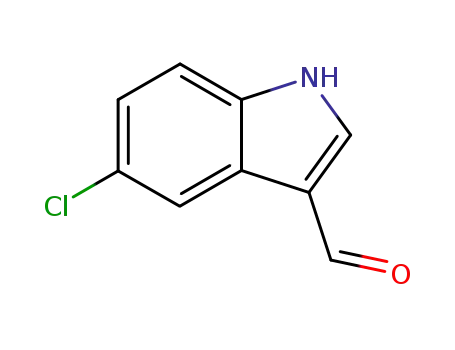
5-chloro-1H-indole-3-carboxaldehyde
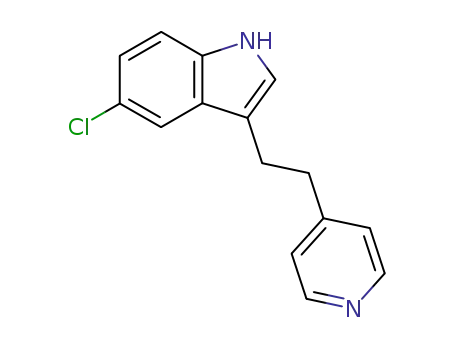
5-chloro-3-<2-(4-pyridyl)ethyl>indole
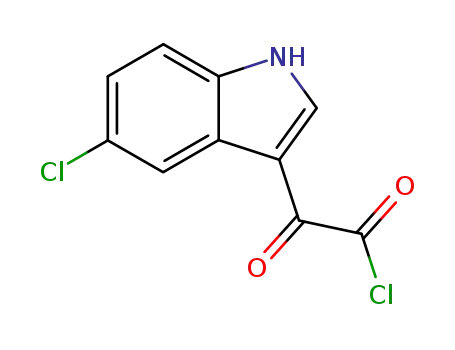
2-(5-chloro-1H-indol-3-yl)-2-oxoacetyl chloride
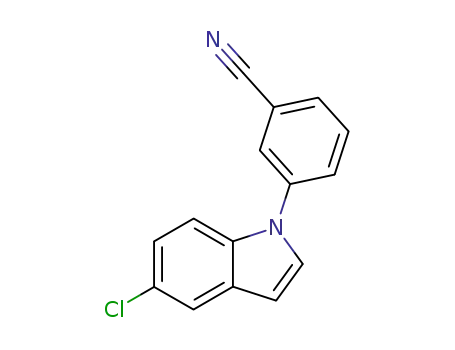
3-(5-Chloro-indol-1-yl)-benzonitrile
CAS:34298-25-4
CAS:22536-67-0
CAS:13750-81-7