Your Location:Home >Products >Intermediates >13750-81-7
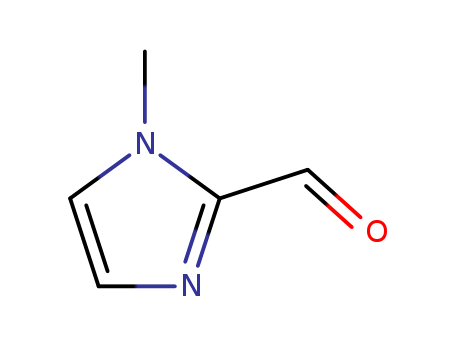

Product Details
|
Chemical Properties |
White to yellow crystalline mass, |
|
Uses |
It is an important raw material and intermediate used in organic synthesis, Pharmaceuticals, agrochemicals and dyestuffs. |
|
Synthesis Reference(s) |
Journal of Medicinal Chemistry, 26, p. 121, 1983 DOI: 10.1021/jm00356a001 |
|
General Description |
1-Methyl-2-imidazolecarboxaldehyde is a heterocyclic building block. It affords tripodal ligands on condensation reaction with tris-(2-aminoethyl)amine (tren). These tripodal ligands react with iron(III) salts in the presence of air to afford iron(II) complexes. Its vibrational spectral studies have been reported. |
InChI:InChI=1/C5H6N2O/c1-7-3-2-6-5(7)4-8/h2-4H,1H3
Bis(N-methylimidazole-2-yl)butadiyne (bm...
Abstract: New heterocyclic privileged me...
A series of 1,3-disubstituted-2-imidazol...
The ligand piperazine-1,4-bis[4-(N-(1-ac...
New tripod ligands containing pyridin-2-...
2-Iodoethynylpyridine and 2-iodoethynyl-...
The increased number of N-atoms induced ...
Provided herein are compounds and pharma...
The invention belongs to the field of me...
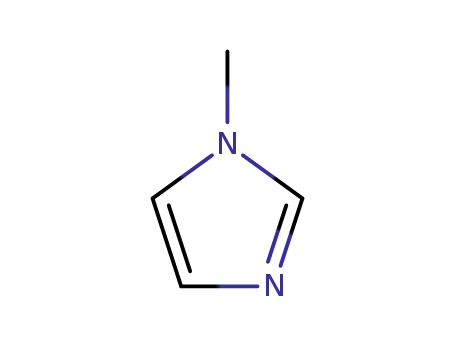
1-methyl-1H-imidazole

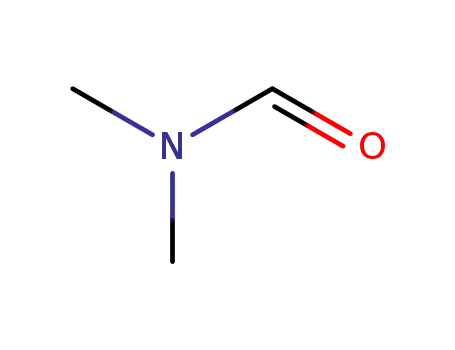
N,N-dimethyl-formamide

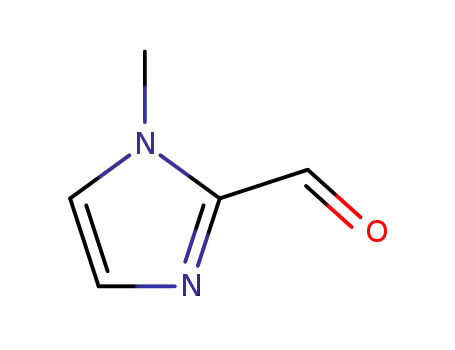
N-methyl-2-imidazolcarboxaldehyde
| Conditions | Yield |
|---|---|
|
1-methyl-1H-imidazole; With n-butyllithium; In tetrahydrofuran; hexane; at -78 - -73 ℃; for 0.0833333h; Inert atmosphere;
N,N-dimethyl-formamide; In tetrahydrofuran; hexane; at -78 - -73 ℃; for 0.0833333h; Inert atmosphere;
With hydrogenchloride; In water; at 20 ℃; for 1h;
|
98.5% |
|
1-methyl-1H-imidazole; With n-butyllithium; In tetrahydrofuran; hexane; at -78 ℃; for 0.0833333h; Inert atmosphere;
N,N-dimethyl-formamide; In tetrahydrofuran; hexane; at -78 ℃; for 1h;
With hydrogenchloride; In tetrahydrofuran; hexane; at 20 ℃; for 1h;
|
97% |
|
With n-butyllithium; In tetrahydrofuran; at -78 - 20 ℃; Inert atmosphere;
|
94% |
|
1-methyl-1H-imidazole; With n-butyllithium; In tetrahydrofuran; hexane; at -78 ℃; for 1h;
N,N-dimethyl-formamide; In tetrahydrofuran; hexane; at -78 - 20 ℃;
|
83% |
|
1-methyl-1H-imidazole; With n-butyllithium; In tetrahydrofuran; at -78 ℃; for 0.5h;
N,N-dimethyl-formamide; at 20 ℃; for 2h;
|
80% |
|
1-methyl-1H-imidazole; With n-butyllithium; In tetrahydrofuran; at 0 ℃; Inert atmosphere;
N,N-dimethyl-formamide; In tetrahydrofuran; at 0 ℃; Inert atmosphere;
|
73% |
|
1-methyl-1H-imidazole; With n-butyllithium; In tetrahydrofuran; at -15 ℃;
N,N-dimethyl-formamide; In tetrahydrofuran; at -15 ℃;
In water; at 20 ℃;
|
72% |
|
1-methyl-1H-imidazole; With n-butyllithium; In tetrahydrofuran; hexane; at -78 ℃; for 1.33333h;
N,N-dimethyl-formamide; In tetrahydrofuran; hexane; at -78 - 20 ℃; for 20h;
|
70% |
|
1-methyl-1H-imidazole; With n-butyllithium; In tetrahydrofuran; at -70 ℃; for 0.5h;
N,N-dimethyl-formamide; In tetrahydrofuran; at -70 - 0 ℃; for 2h;
|
68% |
|
1-methyl-1H-imidazole; With n-butyllithium; In tetrahydrofuran; hexane; at -78 ℃; Inert atmosphere;
N,N-dimethyl-formamide; In tetrahydrofuran; hexane; at -78 - 20 ℃; Inert atmosphere;
|
56% |
|
1-methyl-1H-imidazole; With n-butyllithium; In tetrahydrofuran; hexane; at -78 ℃; for 0.5h; Inert atmosphere;
N,N-dimethyl-formamide; In tetrahydrofuran; hexane; at -78 - 20 ℃; for 1h;
With ammonium chloride; In tetrahydrofuran; hexane; water;
|
52% |
|
1-methyl-1H-imidazole; With n-butyllithium; In tetrahydrofuran; hexane; at -78 ℃; for 0.5h; Inert atmosphere; Darkness;
N,N-dimethyl-formamide; In tetrahydrofuran; hexane; for 0.333333h; Inert atmosphere; Darkness;
|
52% |
|
1-methyl-1H-imidazole; With n-butyllithium; In tetrahydrofuran; at -78 ℃; for 1h; Inert atmosphere;
N,N-dimethyl-formamide; In tetrahydrofuran; at 25 ℃; for 16h;
|
24.8% |
|
With n-butyllithium; Yield given. Multistep reaction; 1.) diethyl ether, from -80 deg C to 0 deg C, 2.) RT, 6 h;
|
|
|
With lithium diisopropyl amide; Yield given. Multistep reaction; 1.) hexane, THF, from -60 deg C to -50 deg C, 3 h, 2.) RT, overnight;
|
|
|
With n-butyllithium; Multistep reaction; 1.) THF, -40 deg C, 2.) THF;
|
|
|
With n-butyllithium; Yield given. Multistep reaction; 1.) THF, hexane, -40 deg C, 1 h, 2.) THF, hexane, RT, 18 h;
|
|
|
1-methyl-1H-imidazole; With n-butyllithium; In diethyl ether; at -78 ℃;
N,N-dimethyl-formamide; In diethyl ether;
|
|
|
With n-butyllithium; In tetrahydrofuran;
|
|
|
With n-butyllithium;
|
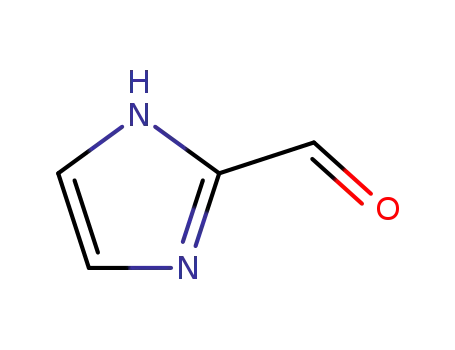
2-imidazolecarbaldehyde

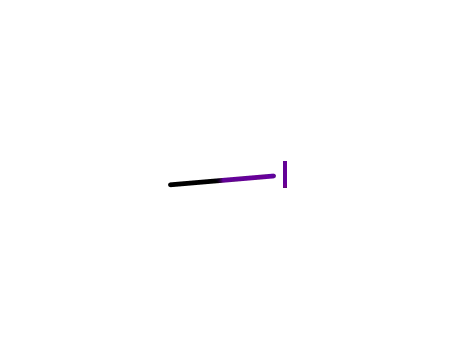
methyl iodide


N-methyl-2-imidazolcarboxaldehyde
| Conditions | Yield |
|---|---|
|
With potassium carbonate; In ethanol; N,N-dimethyl-formamide; at 50 ℃; for 5h;
|
59% |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 50 ℃; for 5h;
|
|
|
With potassium carbonate; In N,N-dimethyl-formamide; at 80 ℃;
|

1-methyl-1H-imidazole
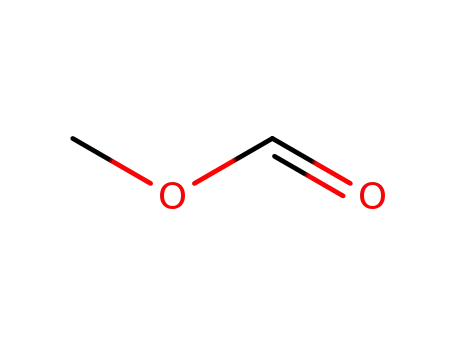
Methyl formate

N,N-dimethyl-formamide
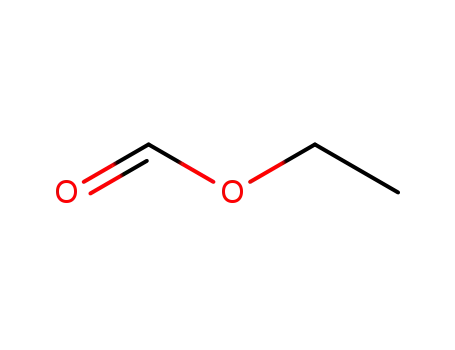
formic acid ethyl ester
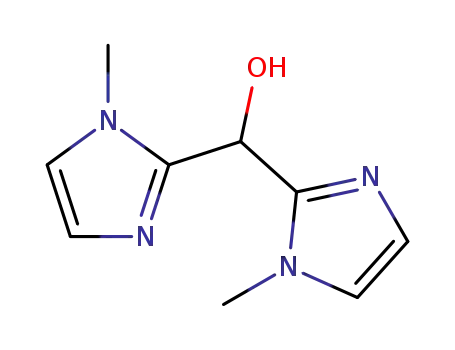
bis(1-methyl-2-imidazolyl)hydroxymethane
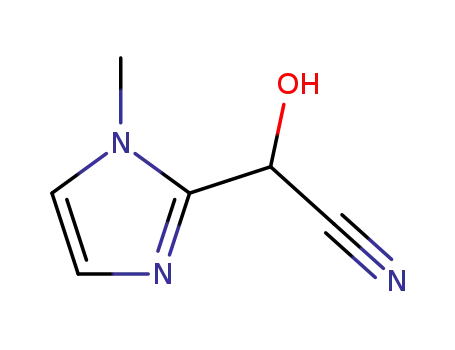
2-hydroxy-2-(1-methyl-2-imidazolyl)acetonitrile
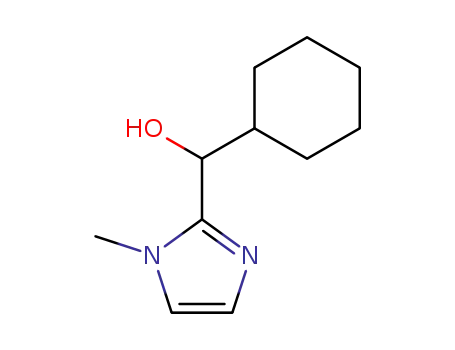
2-(cyclohexylhydroxymethyl)-1-methyl-1H-imidazole
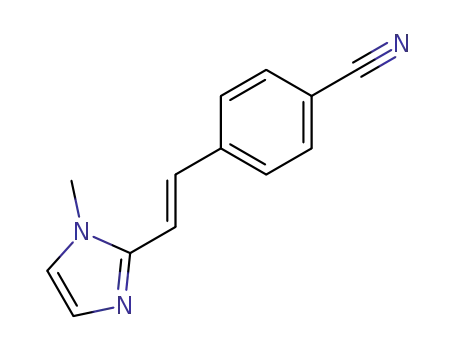
4-[(E)-2-(1-Methyl-1H-imidazol-2-yl)-vinyl]-benzonitrile
CAS:2941-78-8
CAS:1143516-05-5
CAS:17422-32-1
CAS:72-14-0