Your Location:Home >Products >Intermediates >104-53-0
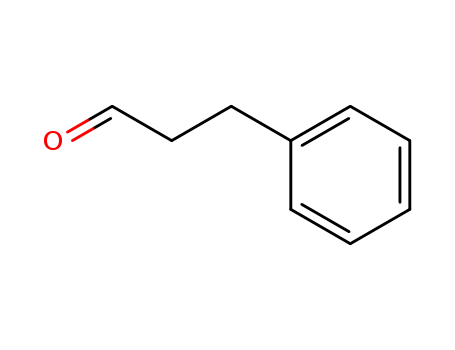
Product Details
|
Chemical Properties |
Pale Yellow Oil |
|
Occurrence |
Reported found in the essential oil of Ceylon cinnamon and in strawberry. Also reported found in tomato, cinnamon, cassia leaf, Gruyere de Comte cheese, beer, cooked trassi, origanum (Spanish) and strawberry. |
|
Uses |
3-Phenylpropanal (cas# 104-53-0) is a compound useful in organic synthesis. |
|
Preparation |
From phenyl propionitrile; also from cinnamic aldehyde diethylacetal. |
|
Taste threshold values |
Taste characteristics at 20 ppm: green, melon, fruity and citrus. |
|
Synthesis Reference(s) |
Chemical and Pharmaceutical Bulletin, 42, p. 1041, 1994 DOI: 10.1248/cpb.42.1041Tetrahedron Letters, 35, p. 1275, 1994 DOI: 10.1016/0040-4039(94)88042-5 |
|
General Description |
Hydrocinnamaldehyde is C=C double bond hydrogenated cinnamaldehyde. It has been synthesized by the selective hydrogenation of C=C double bond of cinnamaldehyde by various methods. Hydrocinnamaldehyde and nitromethane undergoes Henry reaction to form nitroaldols. The C1 and C3 position labelled with 13C of hydrocinnamaldehyde was subjected to mass spectrometry and the fragmentation pattern was elucidated. |
InChI:InChI=1/C9H10O/c10-8-4-7-9-5-2-1-3-6-9/h1-3,5-6,8H,4,7H2
H4Ru4(CO)8L4 (L=PBun3) catalyses selecti...
A novel, unique way to cleave the carbon...
The oxidative deprotection of trimethyls...
-
A series of novel daphneolone analogs wa...
Ultrafine and homogenously dispersed Pt3...
The activity of catalytic systems derive...
An organic-inorganic hybrid material, PK...
Stabilization of transition metals in na...
A series of new chelating diphosphite li...
A Rh phosphine complex was encapsulated ...
-
Perchloric acid adsorbed on silica gel (...
Comparison of the catalytic activity of ...
A tetrameric DABCO-bromine complex was s...
An environmentally benign method for the...
The novel β,γ,δ,ε-unsaturated ketone (2)...
Secondary (E)-allyl vic-diol cleavage in...
The heterogenized versions of [RuCl2(PPh...
Aldehydes are synthesized by hydrogenati...
Abstract: This paper describes the prepa...
-
Pt nanoparticles supported on sheetlike ...
The extraction of the two competing reac...
Herein, it is presented a novel catalyti...
New composites based on the [RhMo6O24H6]...
Carbon homologation reactions occur with...
Novel phosphanylcalix[6]arenes having mo...
Enoate reductases from the family of old...
An efficient procedure for conversion of...
A homogeneously dispersed graphene oxide...
Carbon and nitrogen coated St?ber silica...
Palladium is a key catalyst invaluable t...
The selective hydrogenation of α,β-unsat...
Cycling instability is a persisting prob...
Carbon nanotubes (CNTs) supported Pt cat...
Impressive tolerance is displayed in the...
In this study, our aim is to increase th...
A new method for alcohol oxidation using...
1,2,3-Triazol-5-ylidenes (tzNHC) have be...
Allyldimesitylborane readily yields an a...
Some mono- and disubstituted ethenes hav...
Chemoselective reduction of the C=C bond...
The liquid phase hydrogenation of cinnam...
An enhancement of the catalytic performa...
We report here, for the first time, a si...
Water soluble complexes derived from the...
The rhodium-catalyzed hydroformylation o...
A series of catalysts consisting of ZrO2...
Coordinative bonds have been used to pre...
This work detailed the preparation of a ...
Three arene-ruthenium(II) complexes bear...
Transition-metal-catalyzed branched and ...
Copper hydrides are very useful in hydro...
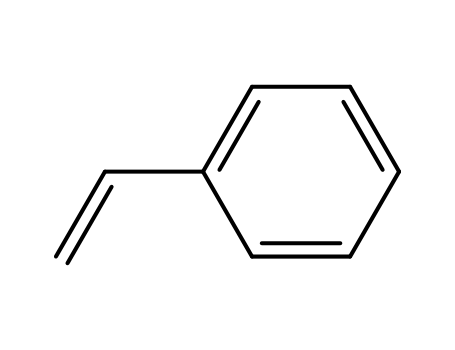
styrene

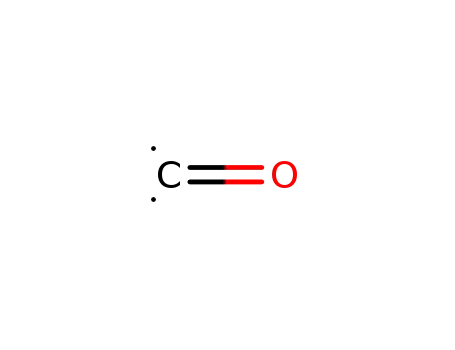
carbon monoxide

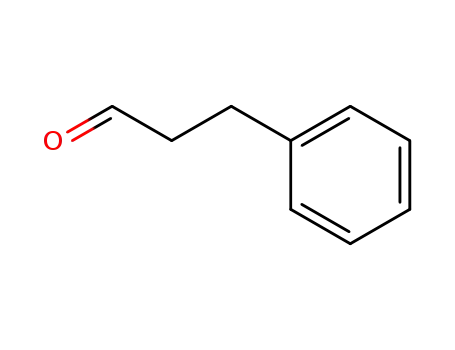
3-phenyl-propionaldehyde

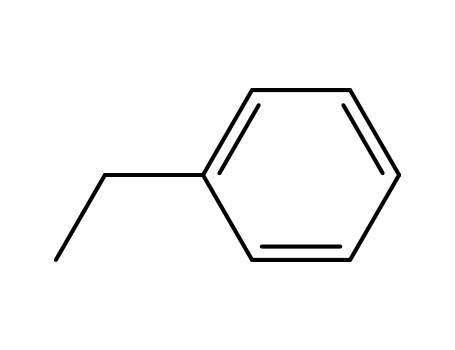
ethylbenzene

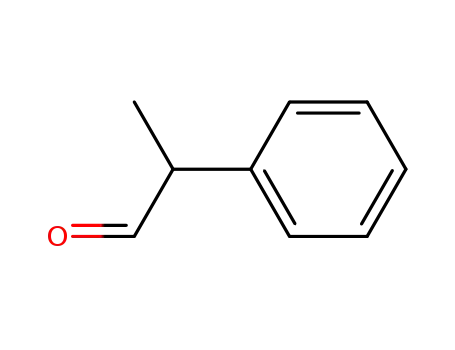
2-Phenylpropanal
| Conditions | Yield |
|---|---|
|
With hydrogen; RhPtuPF6; In toluene; at 80 ℃; under 45600 Torr; Further Variations:; Catalysts; Product distribution;
|
80% 18% 1% |
|
With hydrogen; N-dodecyl-N-(2-hydroxyethyl)-N,N-dimethylammonium bromide; {Rh(cod)[μ-S(CH2)3Si(OMe)3]}2; triphenylphosphine; In water; butan-1-ol; at 80 ℃; for 10h; under 20701.7 Torr; microemulsion/sol-gel;
|
16.4% 73.1% 0.4% |
|
With hydrogen; PtCl2(DIOP); copper dichloride; In toluene; at 100 ℃; for 8h; under 30002.4 Torr; Product distribution; other catalysts, reaction times and temperatures;
|
11% 17% 4% |
|
With bis-diphenylphosphinomethane; platinum; tin(ll) chloride; In toluene; at 100 ℃; for 4h; under 30002.4 Torr; Product distribution; other styrene derivatives; other reagents and catalysts;
|
|
|
With dichloro{(-)-(R,R)-2,2-dimethyl-4,5-bis(diphenylphosphinomethyl)-1,3-dioxolane-P,P'}platinum; hydrogen; In toluene; at 100 ℃; for 72h; under 38000 Torr; Product distribution; also 1-hexene; other catalysts; enantioselectivity of hydroformylation;
|
2.1 % Chromat. |
|
With (PtCl2)2(5,11,17,23-tetra-tert-butyl-25,26,27,28-tetrakis(2-diphenylphosphinoxy-ethoxy)calix<4>arene); hydrogen; tin(ll) chloride; In toluene; at 50 ℃; for 135h; under 60004.8 Torr; Product distribution; hydroformylation with various catalyst-systems;
|
|
|
With hydrogen; tin(ll) chloride; platinum; In toluene; at 60 - 100 ℃; under 60004.8 Torr; Yield given. Yields of byproduct given;
|
|
|
With hydrogen; tin(ll) chloride; chiral (diphosphinite derivative)platinum; In dichloromethane; at 20 ℃; under 15001.2 Torr;
|
|
|
rhodium; under 22501.8 Torr; Further Variations:; Temperatures; Catalysts; Product distribution;
|
|
|
With (S)-N-(2,2'-PPh2)-O-(2,2'-PPh2)-CH2-pyrrolidine; dodecacarbonyltetrarhodium(0); In toluene; at 50 ℃; for 18h; under 9000.72 Torr; Further Variations:; Solvents; Pressures; Reagents; Catalysts; Product distribution;
|
|
|
With [(norbornadiene)rhodium(I)chloride]2; 1-(2',4',6'-tri-isopropylphenyl)-3-methylphosphole; tin(ll) chloride; In toluene; at 100 ℃; for 130h; under 60004.8 Torr; Further Variations:; Reagents; Product distribution;
|
|
|
With hydrogen; C24H25Cl2F3O3PRh*0.5CH2Cl2; In toluene; at 100 ℃; for 2h; under 75006 Torr; Further Variations:; Catalysts; Temperatures; Product distribution;
|
|
|
With 3-(ethoxy-H-phosphonyl)-4-methyl-1-arylphosphole; hydrogen; [(norbornadiene)rhodium(I)chloride]2; In toluene; at 100 ℃; for 2h; under 75006 Torr; Further Variations:; Reagents; Product distribution;
|
80 % Turnov. |
|
With hydrogen; tin(ll) chloride; PdCl2(R,R)-1-Ph2P-2,1'-[(1-(chex)2P)-1,3-Pr-diyl]ferrocene; In toluene; at 90 ℃; for 20h; under 60004.8 Torr; Further Variations:; Temperatures; Catalysts; Product distribution;
|
|
|
With PtCl2(xantphos); hydrogen; tin(ll) chloride; In toluene; at 25 ℃; under 30002.4 Torr; Further Variations:; Temperatures; Pressures; Product distribution;
|
|
|
With trans-[PtCl2(diisopropyl (2-(diphenylphosphino)benzylidene)malonate-P)]; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 24h; under 60006 Torr; regioselective reaction;
|
|
|
styrene; carbon monoxide; With η4-1,5-cyclooctadiene-μ-2,4-pentanedionato-iridium(I); triphenylphosphine; In N-methylpyrrolidone; at 20 - 100 ℃; under 5250.53 Torr; Inert atmosphere; Autoclave;
With hydrogen; In N-methylpyrrolidone; at 100 ℃; for 24h; under 9750.98 Torr; chemoselective reaction; Autoclave;
|
6 %Chromat. |
|
With C24H23Cl3P2Pt; hydrogen; tin(ll) chloride; In toluene; at 50 ℃; for 96h; under 30003 Torr; chemoselective reaction; Autoclave; Inert atmosphere;
|
|
|
With cis-[bis(1-propyl-3-methyl-3-phospholeno)-dichloro-platinum(II)]; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 48h; under 60006 Torr; regioselective reaction; Inert atmosphere;
|
|
|
With C22H22Cl2P2Pt; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 24h; under 67506.8 Torr; regioselective reaction; Autoclave;
|
|
|
With C32H35Cl2NP2Pt; tin(ll) chloride; In toluene; at 60 ℃; for 20h; under 60006 Torr; chemoselective reaction; Inert atmosphere; Autoclave;
|
|
|
With acetylacetonatodicarbonylrhodium(l); 1,3-bis[(diphenylphosphino)oxy]-1,3-diphenylpropane; hydrogen; In toluene; at 80 ℃; for 6h; under 26252.6 Torr; regioselective reaction; Catalytic behavior; Schlenk technique; High pressure;
|
6 %Chromat. |
|
With C42H36ClIrO2P2*C2H3N; hydrogen; In 1,2-dichloro-ethane; at 90 ℃; for 24h; under 18001.8 Torr; Autoclave; Inert atmosphere; Schlenk technique;
|
|
|
With iridium cyclooctadiene acetylacetonate; hydrogen; triphenylphosphine; In 1-methyl-pyrrolidin-2-one; at 100 ℃; for 24h; under 5250.53 - 17251.7 Torr; Autoclave; Inert atmosphere;
|
6 %Chromat. |
|
With rhodium(III) chloride; hydrogen; In tetrahydrofuran; at 120 ℃; for 20h; under 10343.2 Torr; Inert atmosphere; Schlenk technique; Autoclave;
|
|
|
With cis-[bis(1-isopropyl-3-methyl-3-phospholeno)dichloroplatinum(II)]; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 24h; under 60006 Torr; Temperature; regioselective reaction; Inert atmosphere; Autoclave;
|
|
|
With [bis(di-p-tolylphosphinomethyl)cyclohexylamine]dichloroplatinum(II); hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 6h; under 60006 Torr; Reagent/catalyst; Temperature; Time; chemoselective reaction; Inert atmosphere; Autoclave;
|
|
|
With cis-[bis(4-chloro-1-phenyl-5-methyl-1,2,3,6-tetrahydrophosphinino)-dichloroplatinum(II)]; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 10h; under 60006 Torr; Temperature; Time; chemoselective reaction; Catalytic behavior; Inert atmosphere;
|
|
|
With (S)-((4,4’-bi-1,3-benzodioxole)-5,5’-diyl)bis(diphenylphosphine); di-μ-chlorobis(norbornadiene)dirhodium(I); hydrogen; at 60 ℃; for 5h; under 60006 Torr; Reagent/catalyst; Autoclave; Schlenk technique;
|
|
|
With C37H39Cl2NP2Pt; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 3h; Reagent/catalyst; regioselective reaction;
|
|
|
With rhodium contaminated with carbon; hydrogen; at 89.84 ℃; for 12h; under 7500.75 Torr; Catalytic behavior; Autoclave;
|
47 %Chromat. 38 %Chromat. 8 %Chromat. |
|
With hydrogen; In 1,4-dioxane; at 120 ℃; for 12h; under 11251.1 Torr; regioselective reaction; Autoclave;
|

styrene

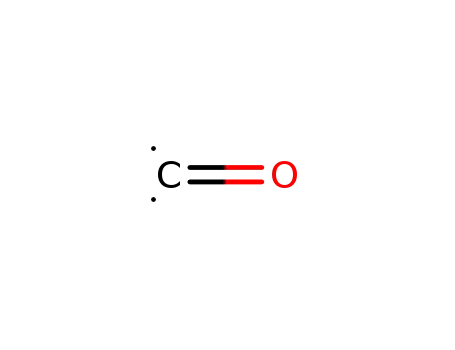
carbon monoxide

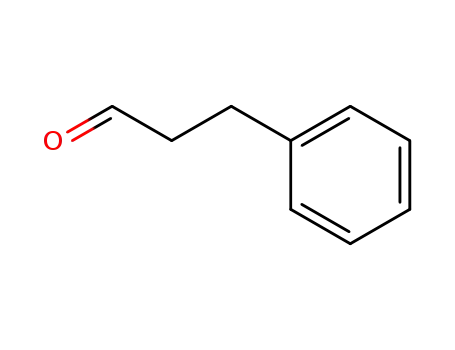
3-phenyl-propionaldehyde


ethylbenzene

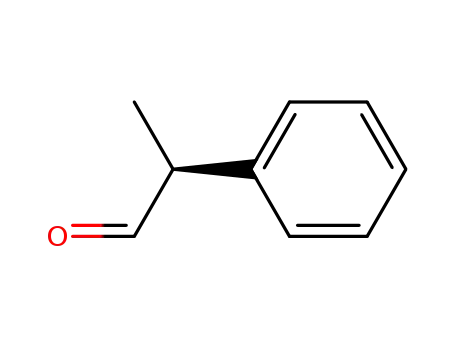
(S)-2-phenyl-propionaldehyde

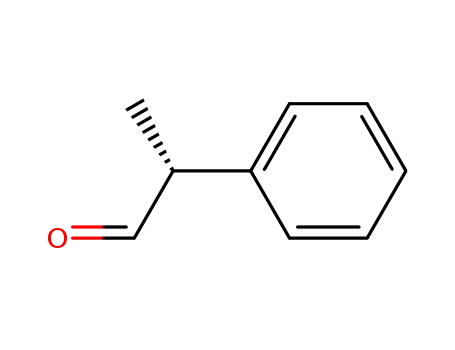
(R)-2-phenylpropanal
| Conditions | Yield |
|---|---|
|
With [(2S,3S)-2,4-bis(diphenylphosphino)pentane]Pt(SnCl3)Cl; hydrogen; In toluene; at 40 ℃; for 138h; under 51714.8 Torr;
|
68.7 % Chromat. 25% 6.3% |
|
With hydrogen; PtCl2
|
|
|
With hydrogen; bis(benzonitrile)dichloroplatinum(II); tin(ll) chloride; (2S,4S)-2,4-bis[((S)-dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-2-yl)oxy]pentane; In toluene; at 100 ℃; for 2h; under 76000 Torr; Title compound not separated from byproducts;
|
|
|
With hydrogen; bis(benzonitrile)dichloroplatinum(II); tin(ll) chloride; (2S,4S)-2,4-bis[((S)-dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-2-yl)oxy]pentane; In toluene; at 17 ℃; for 70h; under 76000 Torr; Title compound not separated from byproducts;
|
|
|
With chiral bis(octahydrodinaphthodioxaphosphepin)-based ligand; hydrogen; tin(ll) chloride; bis(benzonitrile)dichloroplatinum(II); In toluene; at 23 ℃; for 20h; Title compound not separated from byproducts;
|
|
|
With hydrogen; [Rh(NBD)(S)-BINAPO]BF4; In toluene; at 40 ℃; for 15h; under 60004.8 Torr; Further Variations:; Catalysts; Solvents; Temperatures; Product distribution;
|
|
|
With hydrogen; chiral bis(phosphite)PtCl2-SnCl2; In toluene; at 100 ℃; for 2h; under 76000 Torr; Product distribution; other temp., times, solvent and catalyst;
|
|
|
With (2S,4S)-2-(dibenzophospholyl)-4-(diphenylphosphino)pentane; hydrogen; tin(ll) chloride; bis(benzonitrile)dichloroplatinum(II); In toluene; at 24 ℃; for 45h; under 148200 Torr; Further Variations:; Catalysts; Temperatures; Pressures; Kinetics; Product distribution;
|
|
|
With hydrogen; tris(pentafluorophenyl)borate; In toluene; at 100 ℃; for 24h; under 60006 Torr; Title compound not separated from byproducts.;
|
|
|
With C24H23Cl3P2Pt; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 24h; under 30003 Torr; optical yield given as %ee; chemoselective reaction; Autoclave; Inert atmosphere;
|
|
|
With (R(P),R(P))-cis-bis(1-propyl-3-methyl-3-phospholano)-dichloro-platinum(II); hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 96h; under 60006 Torr; optical yield given as %ee; regioselective reaction; Inert atmosphere;
|
|
|
With cis-[bis(1-ethyl-3-methyl-3-phospholeno)-dichloro-platinum(II)]; hydrogen; tin(ll) chloride; In toluene; at 60 ℃; for 72h; under 60006 Torr; Reagent/catalyst; Temperature; Time; enantioselective reaction; Inert atmosphere; Autoclave;
|
29 % ee |
|
With PtCl2{(-)-(2S,4S)-2,4-bis(diphenylphosphino)pentane}; hydrogen; tin(ll) chloride; In toluene; at 60 ℃; for 18h; under 60006 Torr; Temperature; enantioselective reaction; Inert atmosphere; Autoclave;
|
40 % ee |
|
With PtCl2{(-)-(2S,4S)-2,4-bis(diphenylphosphino)pentane}; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 3h; under 60006 Torr; Temperature; enantioselective reaction; Inert atmosphere; Autoclave;
|
7 % ee |
|
With cis-[bis((S)-1-isopropyl-3-methyl-3-phospholeno)dichloroplatinum(II)]; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 24h; under 60006 Torr; Temperature; enantioselective reaction; Inert atmosphere; Autoclave;
|
10 % ee |
|
With cis-[bis((R)-4-chloro-1-phenyl-5-methyl-1,2,3,6-tetrahydrophosphinino)-dichloroplatinum(II)]; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 10h; under 60006 Torr; Temperature; Time; chemoselective reaction; Catalytic behavior; Inert atmosphere;
|
8 % ee |
|
With {(R)-binap}PtCl2; hydrogen; tin(ll) chloride; In toluene; at 40 ℃; for 120h; under 30003 Torr; Temperature; enantioselective reaction; Autoclave; Schlenk technique;
|
32 % ee |
|
With {(R)-binap}PtCl2; hydrogen; tin(ll) chloride; In toluene; at 60 ℃; for 120h; under 60006 Torr; enantioselective reaction; Autoclave; Schlenk technique;
|
16 % ee |
|
With {(R)-binap}PtCl2; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 23h; under 30003 Torr; Temperature; enantioselective reaction; Autoclave; Schlenk technique;
|
24 % ee |
|
With {(R)-binap}PtCl2; hydrogen; tin(ll) chloride; In toluene; at 100 ℃; for 23h; under 60006 Torr; Temperature; enantioselective reaction; Autoclave; Schlenk technique;
|
24 % ee |
|
With (S)-5,5 ′-bis[di(3,5-xylyl)phosphino]-4,4 ′-bi-1,3-benzodioxole; di-μ-chlorobis(norbornadiene)dirhodium(I); hydrogen; at 60 ℃; for 24h; under 60006 Torr; Autoclave; Schlenk technique;
|
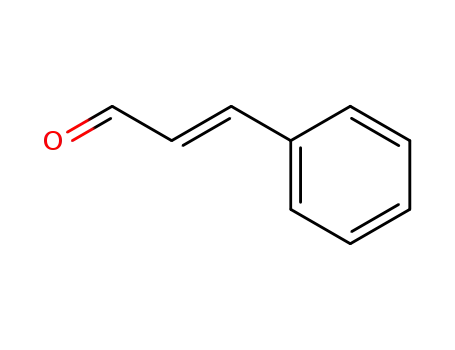
(E)-3-phenylpropenal
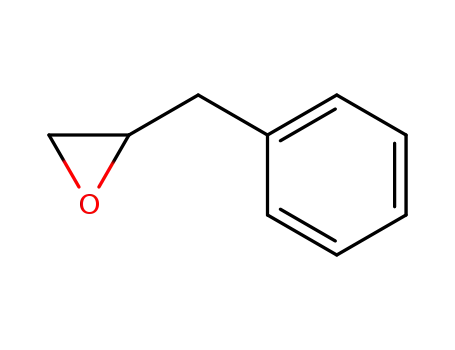
1,2-epoxy-3-phenylpropane
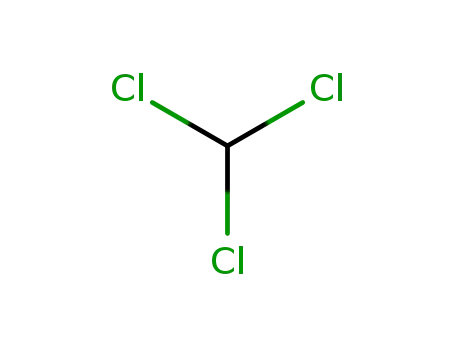
chloroform
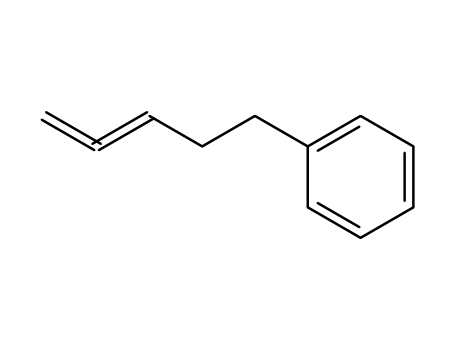
penta-3,4-dienyl-benzene
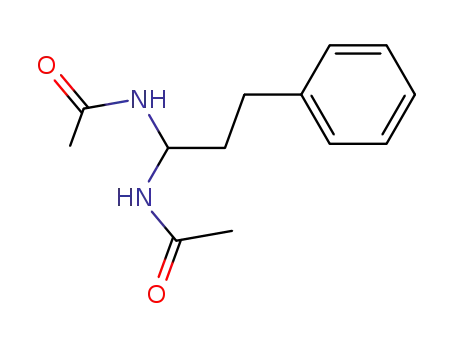
N-(1-acetylamino-3-phenylpropyl)acetamide
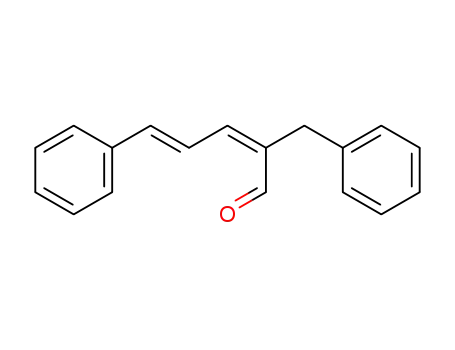
2-benzyl-5-phenyl-penta-2,4-dienal
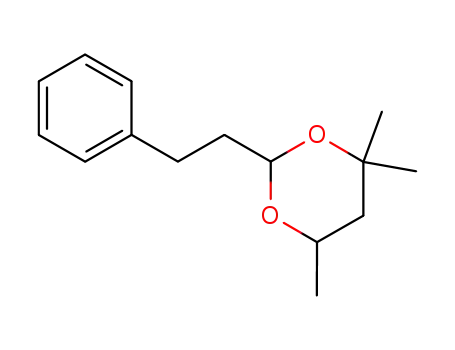
4,4,6-trimethyl-2-phenethyl-[1,3]dioxane
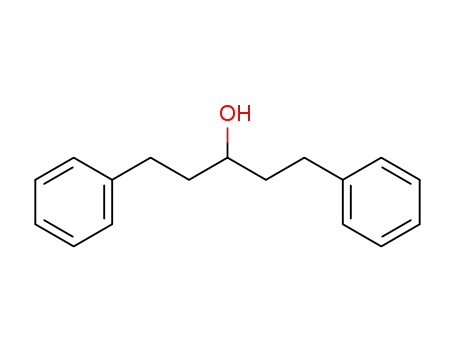
1,5-diphenylpentan-3-ol
CAS:2941-78-8
CAS:1143516-05-5
CAS:53188-07-1
CAS:97-08-5