Your Location:Home >Products >Intermediates >137234-88-9
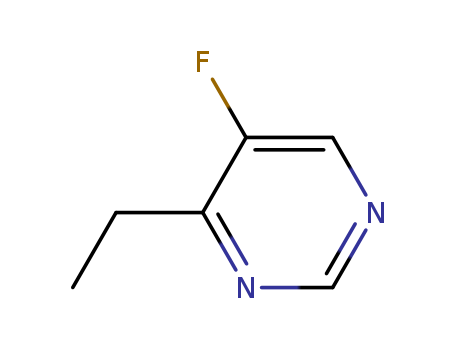

Product Details
|
Uses |
A Voriconazole (V760000) impurity. |
InChI:InChI=1/C7H8FN/c1-2-6-3-4-9-5-7(6)8/h3-5H,2H2,1H3
In the synthesis of (2R,3S)-2-(2,4-diflu...
The invention provide antifungal compoun...
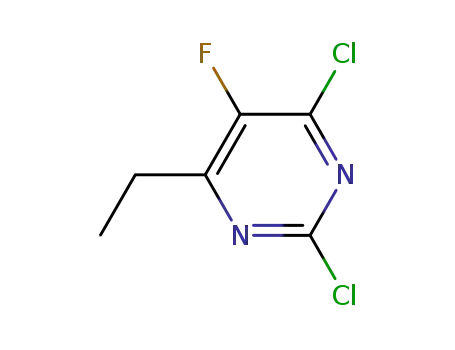
2,4-dichloro-6-ethyl-5-fluoropyrimidine

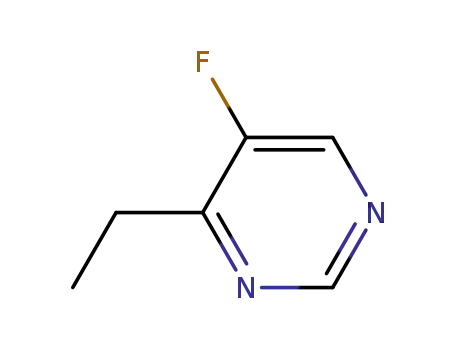
4-ethyl-5-fluoropyrimidine
| Conditions | Yield |
|---|---|
|
With 5%-palladium/activated carbon; hydrogen; sodium acetate; In methanol; at 50 ℃; for 5h; under 2585.81 Torr;
|
35% |
|
With sodium acetate; palladium; In methanol;
|

4-ethyl-5-fluoropyrimidine

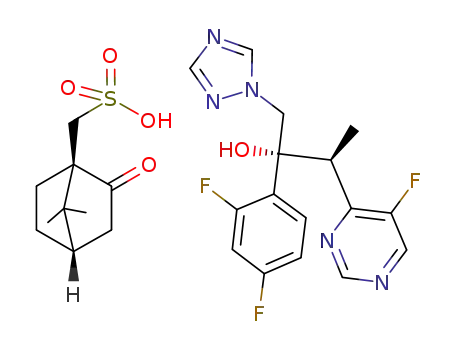
(2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol (1R)-10-camphorsulfonate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: sodium hexamethyldisilazane / tetrahydrofuran; toluene / -60 °C
2: methanol; acetone / Reflux
With sodium hexamethyldisilazane; In tetrahydrofuran; methanol; acetone; toluene;
|
|
|
Multi-step reaction with 3 steps
1: 2,2'-azobis(isobutyronitrile); N-Bromosuccinimide / dichloromethane / 6 h / Reflux
2: zinc; lead / tetrahydrofuran / 16 °C / Inert atmosphere; Reflux
3: methanol; acetone / Reflux
With lead; N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile); zinc; In tetrahydrofuran; methanol; dichloromethane; acetone;
|

2,4-dichloro-6-ethyl-5-fluoropyrimidine
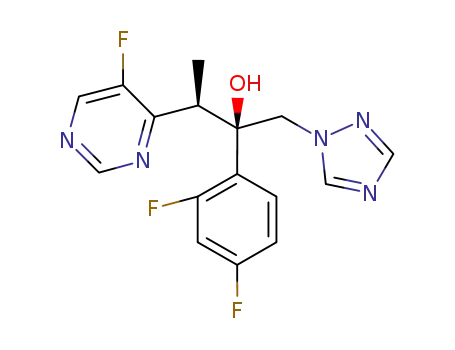
2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol
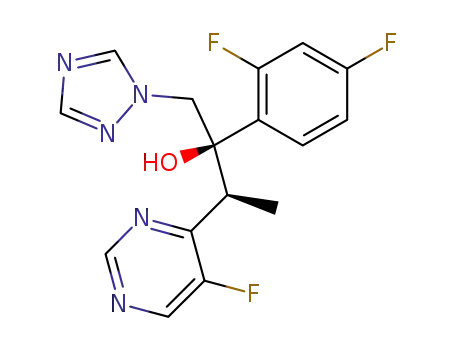
2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butane-2-ol

(2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol (1R)-10-camphorsulfonate
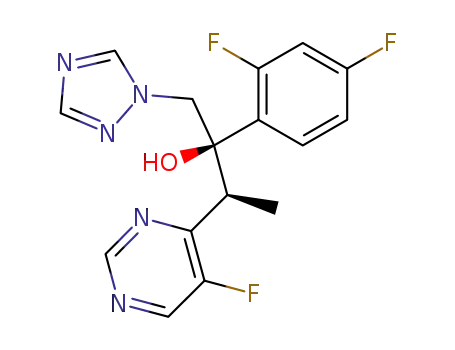
voriconazole
CAS:573762-62-6
CAS:540-63-6
CAS:886-86-2