Your Location:Home >Products >Intermediates >74784-70-6
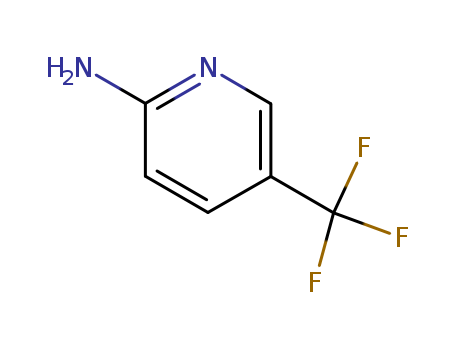

Product Details
|
Chemical Properties |
White to brown solid |
|
Uses |
2-?Amino-?5-?(trifluoromethyl)? is a reagent used in the synthesis of selective inhibitors of urokinase plasminogen activator in a non-cytotoxic form of cancer therapy. |
InChI:InChI=1/C6H5F3N2/c7-6(8,9)4-1-2-5(10)11-3-4/h1-3H,(H2,10,11)
The present invention relates to a catal...
A focused SAR exploration of the lead 4-...
The preparation of the derivatives of 2-...
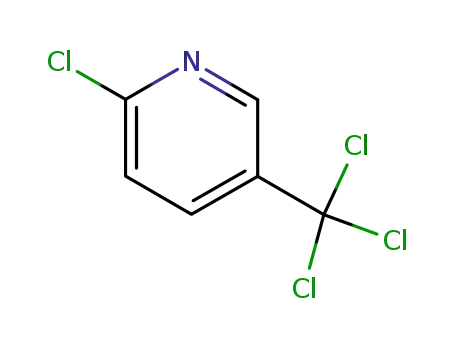
2-chloro-5-(Trichloromethyl)-pyridine

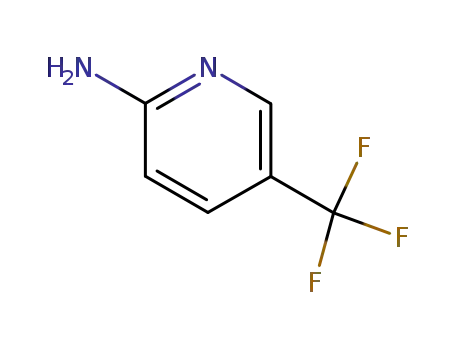
2-amino-5-trifluoromethylpyridine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: 54.5 percent / antimony trifluoride / 0.17 h / Heating
2: 39 percent / aq. ammonia / 8 h / 180 °C
With ammonium hydroxide; antimony(III) fluoride;
|
|
|
Multi-step reaction with 2 steps
1: 54.5 percent / antimony trifluoride / 0.17 h / Heating
2: 75 percent / aq. ammonia / 24 h / 135 °C
With ammonium hydroxide; antimony(III) fluoride;
|

isopropyl benzoate


2-amino-5-trifluoromethylpyridine
| Conditions | Yield |
|---|---|
|
With Zn(2+)*2C2H3O2(1-)*17H2O; at 75 ℃; for 6h;
|
3 %Chromat. |

2-chloro-5-(Trichloromethyl)-pyridine
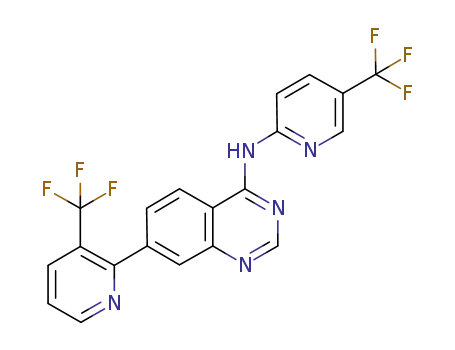
(5-trifluoromethylpyridin-2-yl)-[7-(3-trifluoromethylpyridin-2-yl)quinazolin-4-yl]amine
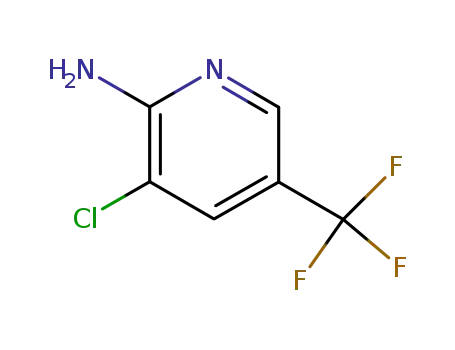
2-amino-3-chloro-5-(trifluoromethyl)pyridine
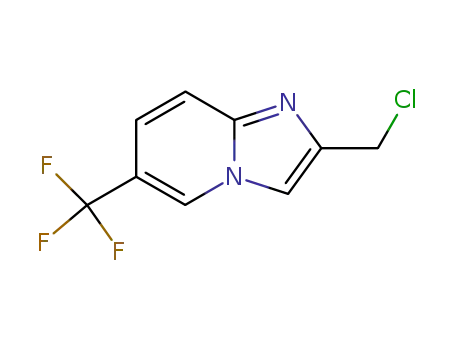
2-(chloromethyl)-6-(trifluoromethyl)imidazo[1,2-a]pyridine
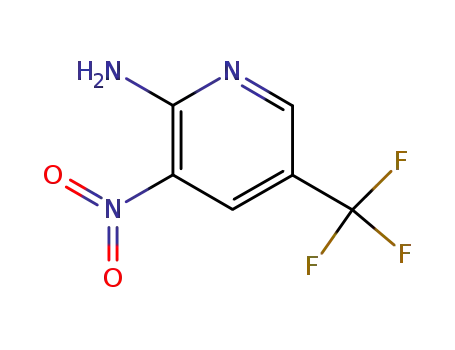
5-(Trifluoromethyl)-3-nitro-2-aminopyridine
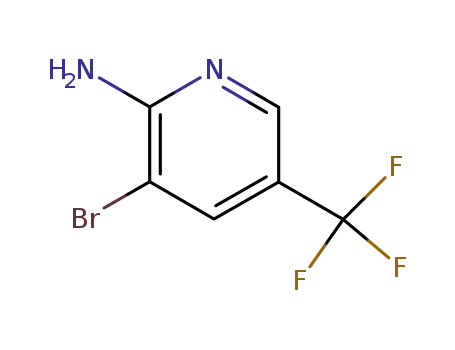
3-bromo-5-trifluoromethyl-pyridin-2-ylamine
CAS:137234-88-9
CAS:615-62-3
CAS:625-05-8