Your Location:Home >Products >API >540737-29-9


Product Details
|
Description |
Tofacitinib citrate is a king of drugs developed by the US pharmaceutical company Pfizer for treating rheumatoid arthritis, trade name Xeljanz, for the treatment of methotrexate inadequate response or intolerance to severe active rheumatoid arthritis (RA) in adult patients. This product is a Janus kinase inhibitor, administered twice daily. November 6, 2012, the US Food and Drug Administration (FDA) and Pfizer jointly announced Tofacitinib citrate is approved for the treatment of methotrexate inadequate response or intolerance to severe active rheumatoid joints arthritis (RA) in adult patients. Xeljanz can be used as monotherapy or in combination with methotrexate or other non-biological disease-modifying antirheumatic drugs (the DMARD) in combination. This medicine should not be in combination with biological DMARD or strong immunosuppressants (such as cyclosporine and azathioprine). Xeljanz is approved by the daily dose of 2 times, each time 5mg. Seven clinical trials evaluated the safety and efficacy of Tofacitinib citrate in moderate to severe active RA in adult patients. In all tests, compared with patients receiving placebo, patients receiving Xeljanz treatment showed significant improvement in clinical response and physical function. In Clinical trials, the most common adverse events were upper respiratory tract infection, headache, diarrhea, nasal congestion, sore throat, and nasopharyngitis. Using Xeljanz was associated with an increased risk of serious infections, including opportunistic infections, tuberculosis, cancer and lymphoma. Xeljanz product label attaches boxed warning on these security risks. Xeljanz treatment is also associated with reducing blood cell counts and increasing cholesterol and liver enzyme values. In order to study Xeljanz long-term impact on heart disease, cancer and severe infections, FDA requires for a post-marketing study, which will evaluate two doses of Xeljanz (Tofacitinib citrate) therapy, and accept a integration of another group of patients approved by the treatment as a control. |
|
In vitro |
CP-690550 is a specific, orally inhibitor of JAK3, it is 20-to 100-fold less potent for JAK2 and JAK1 with IC50 of 20 nM and 112 nM, respectively. CP-690550 doesn't have potent activity against 30 other kinases (all median IC50 > 3000 nM). CP-690,550 inhibits IL-2–induced proliferation with 30-fold greater potency than its effects on GM-CSF–induced proliferation. CP-690550 effectively inhibits a murine mixed lymphocyte reaction (MLR) (IC50 = 91 nM).? CP-690550 potently inhibits IL-4 induced upregulation of CD23 (IC50=57 nM) and class II major histocompatibility complex (MHCII) expression (IC50=71 nM) on murine B cells. A recent research indicates low dose of CP-690550 accelerates the onset of experimental autoimmune encephalomyelitis by potentiating Th17 differentiation. |
|
In vivo |
In a murine model of heterotopic heart transplantation (DBA2 donor heart into C57/BL6 host), CP-690550 results in a dose-dependent increase in survival of transplanted hearts.The EC50 (drug concentration in blood at which 50% of mice will maintain their graft for >28 days) to be ~60 ng/mL.CP-690550 prevents rejection of allogeneic kidneys in nonhuman primate (NHPs, macaca fascicularis) (MST of 62 and 83 days for the 50 to 100 ng/ml groups and 200 to 400 ng/ml groups, respectively). Mice chronically dosed with CP-690550 (1.5-15 mg/kg/day) demonstrate dose and time-dependent alterations in lymphocyte subsets when examined by flow cytometry. The most dramatic change observed is a 96% reduction in splenic NK1.1+TCRb-cell numbers following 21 days of treatment. Delayed-type hypersensitivity (DTH) responses in sensitized mice are reduced in a dose-dependent manner following treatment with CP-690550 (1.87–30 mg/kg, s.c.). |
|
Uses |
Tofacitinib citrate has been used:as a ligand for human serum albumin in fluorescence quenching, dynamic light scattering (DLS) measurements, differential scanning calorimetry and molecular docking studiesas a medium supplement for full depth articular cartilage (FDC) explants to monitor cytokine-induced proteoglycan lossas a Janus kinase inhibitor in MCF7 breast cancer cells |
|
Definition |
ChEBI: A citrate salt obtained by combining equimolar amounts of tofacitinib and citric acid. Used to treat moderately to severely active Rheumatoid Arthritis. |
|
General Description |
Tofacitinib is a synthetic molecule corresponding to a molecular weight of 312.4 Da and is permeable by transcellular diffusion. |
|
Biological Activity |
tofacitinib citrate, also known as cp-690550 citrate, is a potent inhibitor of janus kinase 3 (jak3), a hematopoetic cell-restricted tyrosine kinase involved in signal transduction regulating lymphocyte survival, proliferation, differentiation, and apoptosis. the inhibition is jak3 specific with a selectivity 1000-fold more than other non-jak family kinases. besides inhibiting jaks (ic50 = 1 nm), tofacitinib citrate also inhibits janus kinase 2 (jak2) and janus kinase 1 (jak1) with 20- and 100-fold less in potency respectively. however, in a recent study, the binding affinities (ki) of tofacitinib citrate towards jak1, jak2, and jak3 were reported to be 1.6 nm, 21.7 nm, and 6.5 nm respectively.lalitha vijayakrishnan, r. venkataramanan and palak gulati. treating inflammation with the janus kinase inhibitor cp-690,550. trends in pharmacological sciences 2011: 32 (1); 25-34 |
|
Biochem/physiol Actions |
Tofacitinib is a potent inhibitor of Janus kinase 3 (JAK3) with some JAK-1 inhibitory activity as well. It blocks downstream STAT signaling resulting in potent inhibition of inflammatory cytokines with resultant immunosuppressive and anti-inflammatory activity. Tofacitinib is being investigated for for several autoimmune disorders including, rheumatoid arthritis, psoriasis and dry eye. |
|
Clinical Use |
Potent, selective inhibitor of the Janus kinase family: Treatment of moderate to severe active rheumatoid arthritis |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antibacterials: concentration of tofacitinib reduced by rifampicin - avoid. Antifungals: concentration of tofacitinib increased by fluconazole and ketoconazole - adjust tofacitinib dose. Antipsychotics: increased risk of agranulocytosis with clozapine - avoid. Ciclosporin: concentration of tofacitinib reduced - avoid. Tacrolimus: concentration of tofacitinib reduced - avoid. Vaccines: risk of generalised infections with live vaccines - avoid.ccccccc |
|
Metabolism |
70% metabolised in the liver by CYP3A4 (major) and CYP2C19 (minor). The 8 metabolites produced are inactive. |
InChI:InChI=1S/C16H20N6O.C6H8O7/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16;7-3(8)1-6(13,5(11)12)2-4(9)10/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20);13H,1-2H2,(H,7,8)(H,9,10)(H,11,12)/t11-,13+;/m1./s1
The invention discloses a preparation me...
The invention discloses a tofacitinib ci...
Provided is a process for the preparatio...
The invention relates to the field of me...
![tert-butyl (3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate](/upload/2024/4/ac259fa2-bfa3-45ec-a7bc-f2872b024461.png)
tert-butyl (3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate

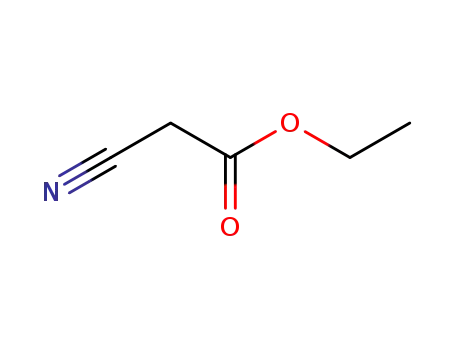
ethyl 2-cyanoacetate

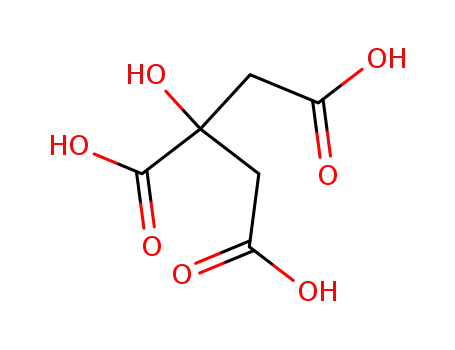
citric acid

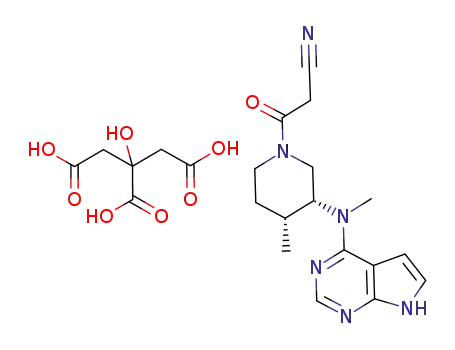
Tofacitinib citrate
| Conditions | Yield |
|---|---|
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In butan-1-ol; at 80 ℃; for 0.166667h;
|
96.4% |
|
tert-butyl (3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate; ethyl 2-cyanoacetate; With 1,8-diazabicyclo[5.4.0]undec-7-ene; In butan-1-ol; at 40 ℃; for 20h; Inert atmosphere;
citric acid; In water; butan-1-ol; at 22 - 81 ℃; for 2.5h; Inert atmosphere;
|
93% |
|
tert-butyl (3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate; ethyl 2-cyanoacetate; With 1,8-diazabicyclo[5.4.0]undec-7-ene; In butan-1-ol; at 35 - 45 ℃; for 7h; Inert atmosphere;
citric acid; In water; butan-1-ol; at 20 - 90 ℃; Inert atmosphere;
|
90.3% |
|
tert-butyl (3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate; In butan-1-ol; at 25 - 30 ℃; for 0.25h; Large scale;
ethyl 2-cyanoacetate; With 1,8-diazabicyclo[5.4.0]undec-7-ene; at 25 - 60 ℃; for 23h; Large scale;
citric acid; In water; butan-1-ol; at 80 - 85 ℃; for 1h; Temperature; Large scale;
|
2.14 kg |
|
tert-butyl (3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate; ethyl 2-cyanoacetate; With Addzyme RD 165G; Hyflo; In tetrahydrofuran; at 65 - 70 ℃; for 68h; Inert atmosphere; Molecular sieve; Enzymatic reaction;
citric acid; In tetrahydrofuran; water; at 0 - 5 ℃; for 3h;
|
11.6 g |
|
tert-butyl (3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate; ethyl 2-cyanoacetate; With pyridine; triethylamine; at 30 ℃; Ionic liquid; Inert atmosphere;
With pyrographite; In water; butan-1-ol; at 80 ℃; for 0.166667h;
citric acid; In water; butan-1-ol; at 60 - 70 ℃; Reagent/catalyst; Temperature;
|
|
|
tert-butyl (3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate; ethyl 2-cyanoacetate; With 1,8-diazabicyclo[5.4.0]undec-7-ene; In butan-1-ol; at 45 ℃; for 12h;
citric acid; In butan-1-ol; at 20 - 40 ℃; for 2h; Time;
|
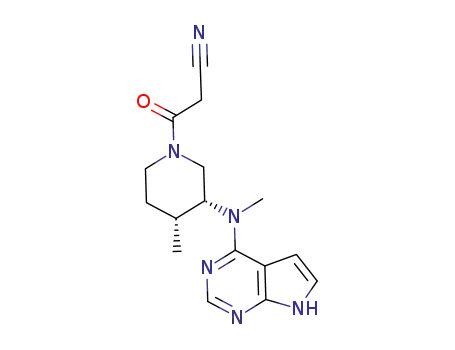
tasocitinib


citric acid


Tofacitinib citrate
| Conditions | Yield |
|---|---|
|
In ethanol; water; at 75 - 85 ℃; for 0.5h;
|
98.5% |
|
In water; acetone; Heating;
|
95% |
|
In propan-1-ol; acetone; at 75 - 80 ℃; for 2h; Solvent;
|
94.3% |
|
In acetone; at 40 ℃; for 2h;
|
92% |
|
In methanol; water; at 40 ℃; for 0.166667h;
|
76% |
|
In acetone; at 20 - 40 ℃; for 24h;
|
75% |
|
In acetone; at 20 ℃; for 2h;
|
|
|
In water; at 70 - 90 ℃; for 1h;
|
19 g |
|
In water; acetone; at 0 - 5 ℃; Solvent;
|
130 g |
|
In methanol; water; at 20 ℃; for 5h; Temperature;
|
0.8 g |
|
In water; butan-1-ol; at 80 ℃; for 1h;
|
|
|
In water; at 96 ℃; Temperature; Large scale;
|
37.6 kg |
|
In methanol; dichloromethane; at 20 - 30 ℃; Large scale;
|
|
|
In water; acetone; for 1h; Reflux; Large scale;
|
3.4 kg |
|
In water; acetone; at 0 - 70 ℃; for 20h; Molecular sieve;
|
29.4 g |
|
In ethanol; water; at 0 ℃;
|
164 g |
|
With 1-hydroxy-pyrrolidine-2,5-dione; N-ethyl-N,N-diisopropylamine; In water; acetonitrile; at 70 - 75 ℃; for 1h; Large scale;
|
2.3 kg |
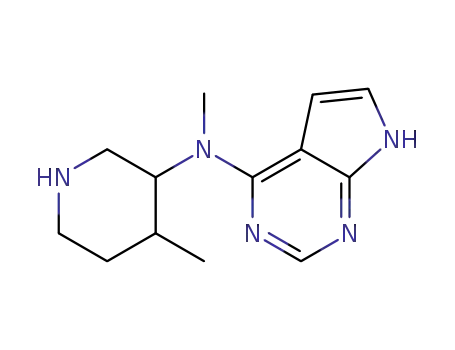
(3R,4R)-methyl-(4-methyl-piperidin-3-yl)-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amine
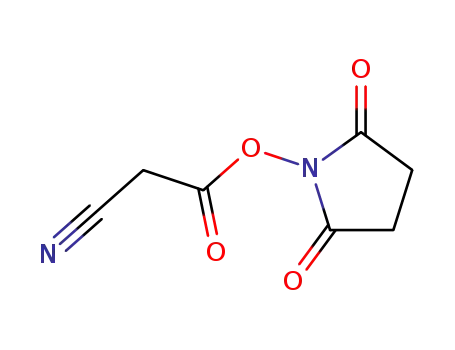
succinimidyl cyanoacetate

citric acid
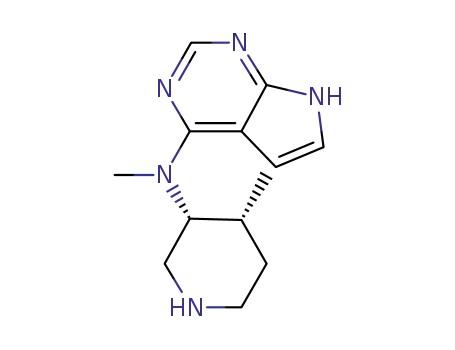
tert-butyl (3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate
CAS:2941-78-8
CAS:1143516-05-5
CAS:97963-62-7
CAS:274693-27-5